Non-Skewed X-inactivation Results in NF-κB Essential Modulator (NEMO) Δ-exon 5-autoinflammatory Syndrome (NEMO-NDAS) in a Female with Incontinentia Pigmenti
- PMID: 39264518
- PMCID: PMC11393190
- DOI: 10.1007/s10875-024-01799-2
Non-Skewed X-inactivation Results in NF-κB Essential Modulator (NEMO) Δ-exon 5-autoinflammatory Syndrome (NEMO-NDAS) in a Female with Incontinentia Pigmenti
Abstract
Purpose: Genetic hypomorphic defects in X chromosomal IKBKG coding for the NF-κB essential modulator (NEMO) lead to ectodermal dysplasia and immunodeficiency in males and the skin disorder incontinentia pigmenti (IP) in females, respectively. NF-κB essential modulator (NEMO) Δ-exon 5-autoinflammatory syndrome (NEMO-NDAS) is a systemic autoinflammatory disease caused by alternative splicing and increased proportion of NEMO-Δex5. We investigated a female carrier presenting with IP and NEMO-NDAS due to non-skewed X-inactivation.
Methods: IKBKG transcripts were quantified in peripheral blood mononuclear cells isolated from the patient, her mother, and healthy controls using RT-PCR and nanopore sequencing. Corresponding proteins were analyzed by western blotting and flow cytometry. Besides toll-like receptor (TLR) and tumor necrosis factor (TNF) signaling, the interferon signature, cytokine production and X-inactivation status were investigated.
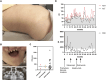
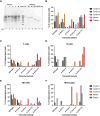
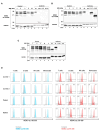
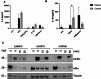
Results: IP and autoinflammation with recurrent fever, oral ulcers, hepatitis, and neutropenia, but no immunodeficiency was observed in a female patient. Besides moderately reduced NEMO signaling function, type I interferonopathy, and elevated IL-18 and CXCL10 were found. She and her mother both carried the heterozygous variant c.613 C > T p.(Gln205*) in exon 5 of IKBKG previously reported in NEMO-deficient patients. However, X-inactivation was skewed in the mother, but not in the patient. Alternative splicing led to increased ratios of NEMO-Dex5 over full-length protein in peripheral blood cell subsets causing autoinflammation. Clinical symptoms partially resolved under treatment with TNF inhibitors.
Conclusion: Non-skewed X-inactivation can lead to NEMO-NDAS in females with IP carrying hypomorphic IKBKG variants due to alternative splicing and increased proportions of NEMO-∆ex5.
Keywords: Autoinflammation; Immunodeficiency; Incontinentia pigmenti; NEMO; Non-skewed X-inactivation.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Immunodeficiency in Two Female Patients with Incontinentia Pigmenti with Heterozygous NEMO Mutation Diagnosed by LPS Unresponsiveness.J Clin Immunol. 2017 Aug;37(6):529-538. doi: 10.1007/s10875-017-0417-3. Epub 2017 Jul 12. J Clin Immunol. 2017. PMID: 28702714
-
Lack of interaction between NEMO and SHARPIN impairs linear ubiquitination and NF-κB activation and leads to incontinentia pigmenti.J Allergy Clin Immunol. 2017 Dec;140(6):1671-1682.e2. doi: 10.1016/j.jaci.2016.11.056. Epub 2017 Feb 27. J Allergy Clin Immunol. 2017. PMID: 28249776
-
An Atypical Incontinentia Pigmenti Female with Persistent Mucocutaneous Hyperinflammation and Immunodeficiency Caused by a Novel Germline IKBKG Missense Mutation.J Clin Immunol. 2023 Nov;43(8):2165-2180. doi: 10.1007/s10875-023-01564-x. Epub 2023 Oct 13. J Clin Immunol. 2023. PMID: 37831401
-
EDA-ID and IP, two faces of the same coin: how the same IKBKG/NEMO mutation affecting the NF-κB pathway can cause immunodeficiency and/or inflammation.Int Rev Immunol. 2015;34(6):445-59. doi: 10.3109/08830185.2015.1055331. Epub 2015 Aug 13. Int Rev Immunol. 2015. PMID: 26269396 Review.
-
Incontinentia pigmenti (Bloch-Sulzberger syndrome).Handb Clin Neurol. 2015;132:271-80. doi: 10.1016/B978-0-444-62702-5.00020-2. Handb Clin Neurol. 2015. PMID: 26564087 Review.
Cited by
-
Enterokinase deficiency associated with novel TMPRSS15 gene mutations: a case report.Front Pediatr. 2025 Jan 29;13:1484208. doi: 10.3389/fped.2025.1484208. eCollection 2025. Front Pediatr. 2025. PMID: 39944319 Free PMC article.
-
Partial Loss of NEMO Function in a Female Carrier with No Incontinentia Pigmenti.J Clin Med. 2025 Jan 9;14(2):363. doi: 10.3390/jcm14020363. J Clin Med. 2025. PMID: 39860371 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

