Simultaneous Targeting of NQO1 and SOD1 Eradicates Breast Cancer Stem Cells via Mitochondrial Futile Redox Cycling
- PMID: 39264695
- PMCID: PMC11647209
- DOI: 10.1158/0008-5472.CAN-24-0800
Simultaneous Targeting of NQO1 and SOD1 Eradicates Breast Cancer Stem Cells via Mitochondrial Futile Redox Cycling
Abstract
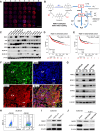
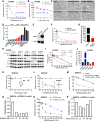
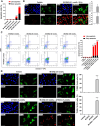
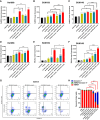
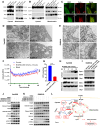
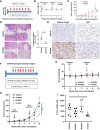
Triple-negative breast cancer (TNBC) contains the highest proportion of cancer stem-like cells (CSC), which display intrinsic resistance to currently available cancer therapies. This therapeutic resistance is partially mediated by an antioxidant defense coordinated by the transcription factor NRF2 and its downstream targets that include NAD(P)H quinone oxidoreductase 1 (NQO1). In this study, we identified the antioxidant enzymes NQO1 and superoxide dismutase 1 (SOD1) as therapeutic vulnerabilities of ALDH+ epithelial-like CSCs and CD24-/loCD44+/hi mesenchymal-like CSCs in TNBC. Effective targeting of these CSC states was achieved by using isobutyl-deoxynyboquinone (IB-DNQ), a potent and specific NQO1-bioactivatable futile redox cycling molecule, which generated large amounts of reactive oxygen species including superoxide and hydrogen peroxide. Furthermore, the CSC killing effect was specifically enhanced by genetic or pharmacologic inhibition of SOD1, a copper-containing superoxide dismutase highly expressed in TNBC. Mechanistically, a significant portion of NQO1 resides in the mitochondrial intermembrane space, catalyzing futile redox cycling from IB-DNQ to generate high levels of mitochondrial superoxide, and SOD1 inhibition markedly potentiated this effect, resulting in mitochondrial oxidative injury, cytochrome c release, and activation of the caspase-3-mediated apoptotic pathway. Treatment with IB-DNQ alone or together with SOD1 inhibition effectively suppressed tumor growth, metastasis, and tumor-initiating potential in xenograft models of TNBC expressing different levels of NQO1. This futile oxidant-generating strategy, which targets CSCs across the epithelial-mesenchymal continuum, could be a promising therapeutic approach for treating patients with TNBC. Significance: Combining NQO1-bioactivatable futile oxidant generators with SOD1 inhibition eliminates breast cancer stem cells, providing a therapeutic strategy that may have wide applicability, as NQO1 and SOD1 are overexpressed in several cancers.
©2024 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
P.J. Hergenrother reports personal fees from Vanquish Oncology, Systems Oncology, Oncoteq, and Arrepath outside the submitted work, as well as US patent 9233960 issued. No disclosures were reported by the other authors.
Figures

Similar articles
-
Isopentyl-Deoxynboquinone Induces Mitochondrial Dysfunction and G2/M Phase Cell Cycle Arrest to Selectively Kill NQO1-Positive Pancreatic Cancer Cells.Antioxid Redox Signal. 2024 Jul;41(1-3):74-92. doi: 10.1089/ars.2022.0224. Epub 2024 Jan 8. Antioxid Redox Signal. 2024. PMID: 37950707 Free PMC article.
-
An update on cancer stem cell survival pathways involved in chemoresistance in triple-negative breast cancer.Future Oncol. 2025 Mar;21(6):715-735. doi: 10.1080/14796694.2025.2461443. Epub 2025 Feb 12. Future Oncol. 2025. PMID: 39936282 Review.
-
Amino acid transporter LAT1 (SLC7A5) promotes metabolic rewiring in TNBC progression through the L-Trp/QPRT/NAD+ pathway.J Exp Clin Cancer Res. 2025 Jul 3;44(1):190. doi: 10.1186/s13046-025-03446-z. J Exp Clin Cancer Res. 2025. PMID: 40611146 Free PMC article.
-
Metabolic Targeting of Oxidative Phosphorylation Enhances Chemosensitivity in Triple-Negative Breast Cancer via a Synergistic Nanomedicine.Theranostics. 2025 Jun 23;15(15):7607-7626. doi: 10.7150/thno.116250. eCollection 2025. Theranostics. 2025. PMID: 40756342 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
Cited by
-
Unraveling systemic responses to NQO1-activated IB-DNQ and Rucaparib single and dual agent therapy in triple-negative breast cancers.bioRxiv [Preprint]. 2025 Apr 8:2024.05.15.594427. doi: 10.1101/2024.05.15.594427. bioRxiv. 2025. PMID: 38798459 Free PMC article. Preprint.
-
Role of Oxidative Stress in the Occurrence, Development, and Treatment of Breast Cancer.Antioxidants (Basel). 2025 Jan 17;14(1):104. doi: 10.3390/antiox14010104. Antioxidants (Basel). 2025. PMID: 39857438 Free PMC article. Review.
References
-
- Abdoli Shadbad M, Hosseinkhani N, Asadzadeh Z, Derakhshani A, Karim Ahangar N, Hemmat N, et al. . A systematic review to clarify the prognostic values of CD44 and CD44+CD24− phenotype in triple-negative breast cancer patients: lessons learned and the road ahead. Front Oncol 2021;11:689839. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

