Postprandial metabolomics analysis reveals disordered serotonin metabolism in post-bariatric hypoglycemia
- PMID: 39264731
- PMCID: PMC11527454
- DOI: 10.1172/JCI180157
Postprandial metabolomics analysis reveals disordered serotonin metabolism in post-bariatric hypoglycemia
Abstract
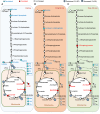
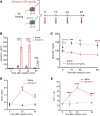
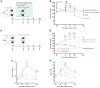
BACKGROUNDBariatric surgery is a potent therapeutic approach for obesity and type 2 diabetes but can be complicated by post-bariatric hypoglycemia (PBH). PBH typically occurs 1-3 hours after meals, in association with exaggerated postprandial levels of incretins and insulin.METHODSTo identify mediators of disordered metabolism in PBH, we analyzed the plasma metabolome in the fasting state and 30 and 120 minutes after mixed meal in 3 groups: PBH (n = 13), asymptomatic post-Roux-en-Y gastric bypass (post-RYGB) (n = 10), and nonsurgical controls (n = 8).RESULTSIn the fasting state, multiple tricarboxylic acid cycle intermediates and the ketone β-hydroxybutyrate were increased by 30%-80% in PBH versus asymptomatic. Conversely, multiple amino acids (branched-chain amino acids, tryptophan) and polyunsaturated lipids were reduced by 20%-50% in PBH versus asymptomatic. Tryptophan-related metabolites, including kynurenate, xanthurenate, and serotonin, were reduced 2- to 10-fold in PBH in the fasting state. Postprandially, plasma serotonin was uniquely increased 1.9-fold in PBH versus asymptomatic post-RYGB. In mice, serotonin administration lowered glucose and increased plasma insulin and GLP-1. Moreover, serotonin-induced hypoglycemia in mice was blocked by the nonspecific serotonin receptor antagonist cyproheptadine and the specific serotonin receptor 2 antagonist ketanserin.CONCLUSIONTogether these data suggest that increased postprandial serotonin may contribute to the pathophysiology of PBH and provide a potential therapeutic target.FUNDINGNational Institutes of Health (NIH) grant R01-DK121995, NIH grant P30-DK036836 (Diabetes Research Center grant, Joslin Diabetes Center), and Fundação de Amparo à Pesquisa do Estado de São Paulo grant 2018/22111-2.
Keywords: Endocrinology; Glucose metabolism; Metabolism.
Conflict of interest statement
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

