A positive cytokine/chemokine feedback loop establishes plasmacytoid DC-driven autoimmune pancreatitis in IgG4-related disease
- PMID: 39264798
- PMCID: PMC11529986
- DOI: 10.1172/jci.insight.167910
A positive cytokine/chemokine feedback loop establishes plasmacytoid DC-driven autoimmune pancreatitis in IgG4-related disease
Abstract
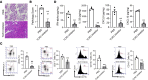
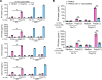
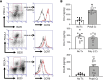
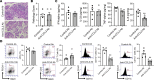
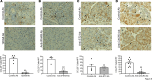
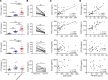
The pathogenesis of the murine model of autoimmune pancreatitis associated with IgG4-related disease (AIP/IgG4-RD) induced by administration of polyinosinic-polycytidylic acid (poly[I:C]) is incompletely understood. While it is known that murine and human AIP/IgG4-RD is driven by plasmacytoid dendritic cells (pDCs) producing IFN-α, the origin of these cells and their relation to effector T cells is not known. Here, we show that murine AIP was initiated by TLR3-bearing conventional DCs in the uninflamed pancreas whose activation by the TLR3 ligand poly(I:C) caused IFN-α, CXCL9, and CXCL10 secretion. This, in turn, induced pancreatic recruitment of CXCR3+ T cells and these T cells, via their secretion of CCL25, facilitated migration of pDCs bearing CCR9 into the pancreas. This established a feedback loop anchored by the now dominant pDC production of IFN-α and the continued CXCR3+ T cell facilitation of pDC migration. Remarkably, the interaction between CXCR3+ T cells and pDCs also existed at the functional level since this interaction enhanced the production of CCL25 and IFN-α by CXCR3+ T cells and pDCs, respectively. Evidence presented here that a similar disease mechanism was present in human AIP/IgG4-RD creates new avenues of disease treatment.
Keywords: Autoimmunity; Chemokines; Cytokines; Gastroenterology.
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

