CETZOLE Analogs as Potent Ferroptosis Inducers and Their Target Identification Using Covalent/Affinity Probes
- PMID: 39264826
- PMCID: PMC12338762
- DOI: 10.1021/acs.jmedchem.3c02084
CETZOLE Analogs as Potent Ferroptosis Inducers and Their Target Identification Using Covalent/Affinity Probes
Abstract
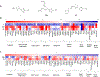
Ferroptosis is a recently discovered cell death mechanism triggered by iron-dependent elevation of reactive oxygen species leading to lipid membrane peroxidation. We previously reported the development of a new class of ferroptosis inducers referred to as CETZOLEs with CC50 values in the low micromolar range. Structure-activity relationship study of these compounds led to the development of more potent analogs with CC50 values in the nanomolar range. Cells exposed to these compounds displayed the hallmarks of ferroptosis including cell death through ROS accumulation. Cancer cells were found to be more sensitive to these compounds than normal cells. Proteomic studies using covalent and affinity probes led to the identification of cystathionine β-synthase, peroxiredoxins, ADP/ATP carriers, and glucose dehydrogenase as enriched proteins. The binding of CETZOLEs to these proteins as well as GPX4 was validated by Western blotting. This group of proteins is known to be associated with cellular antioxidant pathways.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
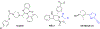





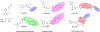


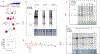
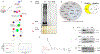
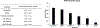


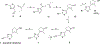



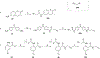
Similar articles
-
Aldo-keto Reductase 1B10 (AKR1B10) Suppresses Sensitivity of Ferroptosis in TNBC by Activating the AKT/GSK3β/Nrf2/GPX4 Axis.Front Biosci (Landmark Ed). 2025 Jun 27;30(6):36615. doi: 10.31083/FBL36615. Front Biosci (Landmark Ed). 2025. PMID: 40613296
-
Hydroxytyrosol induced ferroptosis through Nrf2 signaling pathway in colorectal cancer cells.Sci Rep. 2025 Jul 1;15(1):21271. doi: 10.1038/s41598-025-04415-4. Sci Rep. 2025. PMID: 40594119 Free PMC article.
-
Design and synthesis of Sorafenib analogues and evaluation of their ferroptosis-inducing effects in tumor cells.Bioorg Med Chem. 2025 Oct 1;128:118255. doi: 10.1016/j.bmc.2025.118255. Epub 2025 May 22. Bioorg Med Chem. 2025. PMID: 40440788
-
Targeting ferroptosis using Chinese herbal compounds to treat respiratory diseases.Phytomedicine. 2024 Jul 25;130:155738. doi: 10.1016/j.phymed.2024.155738. Epub 2024 Jun 1. Phytomedicine. 2024. PMID: 38824825
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
References
-
- American Cancer Society. Facts & Figures. Ca-Cancer J. Clin. 2019, 76.
-
- Siegel RL; Miller KD; Fuchs HE; Jemal A Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71 (1), 7–33. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

