Lactate supports Treg function and immune balance via MGAT1 effects on N-glycosylation in the mitochondria
- PMID: 39264847
- PMCID: PMC11473165
- DOI: 10.1172/JCI175897
Lactate supports Treg function and immune balance via MGAT1 effects on N-glycosylation in the mitochondria
Abstract
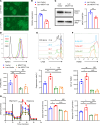
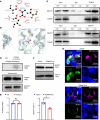
Current research reports that lactate affects Treg metabolism, although the precise mechanism has only been partially elucidated. In this study, we presented evidence demonstrating that elevated lactate levels enhanced cell proliferation, suppressive capabilities, and oxidative phosphorylation (OXPHOS) in human Tregs. The expression levels of Monocarboxylate Transporters 1/2/4 (MCT1/2/4) regulate intracellular lactate concentration, thereby influencing the varying responses observed in naive Tregs and memory Tregs. Through mitochondrial isolation, sequencing, and analysis of human Tregs, we determined that α-1,3-Mannosyl-Glycoprotein 2-β-N-Acetylglucosaminyltransferase (MGAT1) served as the pivotal driver initiating downstream N-glycosylation events involving progranulin (GRN) and hypoxia-upregulated 1 (HYOU1), consequently enhancing Treg OXPHOS. The mechanism by which MGAT1 was upregulated in mitochondria depended on elevated intracellular lactate that promoted the activation of XBP1s. This, in turn, supported MGAT1 transcription as well as the interaction of lactate with the translocase of the mitochondrial outer membrane 70 (TOM70) import receptor, facilitating MGAT1 translocation into mitochondria. Pretreatment of Tregs with lactate reduced mortality in a xenogeneic graft-versus-host disease (GvHD) model. Together, these findings underscored the active regulatory role of lactate in human Treg metabolism through the upregulation of MGAT1 transcription and its facilitated translocation into the mitochondria.
Keywords: Immunology; Metabolism; T cells.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

