The final walk with preptin
- PMID: 39264940
- PMCID: PMC11392399
- DOI: 10.1371/journal.pone.0309726
The final walk with preptin
Abstract
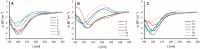
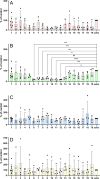
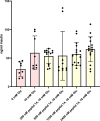
Preptin, a 34-amino acid peptide derived from pro-IGF2, is believed to influence various physiological processes, including insulin secretion and the regulation of bone metabolism. Despite its recognized involvement, the precise physiological role of preptin remains enigmatic. To address this knowledge gap, we synthesized 16 analogs of preptin, spanning a spectrum from full-length forms to fragments, and conducted comprehensive comparative activity evaluations alongside native human, mouse and rat preptin. Our study aimed to elucidate the physiological role of preptin. Contrary to previous indications of broad biological activity, our thorough analyses across diverse cell types revealed no significant biological activity associated with preptin or its analogs. This suggests that the associations of preptin with various diseases or tissue-specific abundance fluctuations may be influenced by factors beyond preptin itself, such as higher levels of IGF2 or IGF2 proforms present in tissues. In conclusion, our findings challenge the conventional notion of preptin as an isolated biologically active molecule and underscore the complexity of its interactions within biological systems. Rather than acting independently, the observed effects of preptin may arise from experimental conditions, elevated preptin concentrations, or interactions with related molecules such as IGF2.
Copyright: © 2024 Mrázková et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




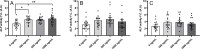

References
-
- Chrétien M, Mbikay M. 60 year of POMC From the prohormone theory to pro-opiomelanocortin and to proprotein convertases (PCSK1 to PCSK9). J Mol Endocrinol. 2016;56(4):T49–T62. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

