Growth on stiffer substrates impacts animal health and longevity in C. elegans
- PMID: 39264947
- PMCID: PMC11392421
- DOI: 10.1371/journal.pone.0302673
Growth on stiffer substrates impacts animal health and longevity in C. elegans
Abstract
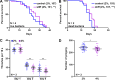
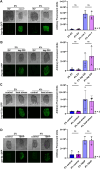
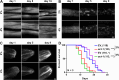
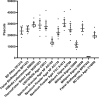
Mechanical stress is a measure of internal resistance exhibited by a body or material when external forces, such as compression, tension, bending, etc. are applied. The study of mechanical stress on health and aging is a continuously growing field, as major changes to the extracellular matrix and cell-to-cell adhesions can result in dramatic changes to tissue stiffness during aging and diseased conditions. For example, during normal aging, many tissues including the ovaries, skin, blood vessels, and heart exhibit increased stiffness, which can result in a significant reduction in function of that organ. As such, numerous model systems have recently emerged to study the impact of mechanical and physical stress on cell and tissue health, including cell-culture conditions with matrigels and other surfaces that alter substrate stiffness and ex vivo tissue models that can apply stress directly to organs like muscle or tendons. Here, we sought to develop a novel method in an in vivo model organism setting to study the impact of altering substrate stiffness on aging by changing the stiffness of solid agar medium used for growth of C. elegans. We found that greater substrate stiffness had limited effects on cellular health, gene expression, organismal health, stress resilience, and longevity. Overall, our study reveals that altering substrate stiffness of growth medium for C. elegans has only mild impact on animal health and longevity; however, these impacts were not nominal and open up important considerations for C. elegans biologists in standardizing agar medium choice for experimental assays.
Copyright: © 2024 Oorloff et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Update of
-
Mechanical stress through growth on stiffer substrates impacts animal health and longevity in C. elegans.bioRxiv [Preprint]. 2024 Apr 12:2024.04.11.589121. doi: 10.1101/2024.04.11.589121. bioRxiv. 2024. Update in: PLoS One. 2024 Sep 12;19(9):e0302673. doi: 10.1371/journal.pone.0302673. PMID: 38645203 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

