Evaluation of hydroxychloroquine or chloroquine for the prevention of COVID-19 (COPCOV): A double-blind, randomised, placebo-controlled trial
- PMID: 39264960
- PMCID: PMC11392261
- DOI: 10.1371/journal.pmed.1004428
Evaluation of hydroxychloroquine or chloroquine for the prevention of COVID-19 (COPCOV): A double-blind, randomised, placebo-controlled trial
Abstract
Background: Hydroxychloroquine (HCQ) has proved ineffective in treating patients hospitalised with Coronavirus Disease 2019 (COVID-19), but uncertainty remains over its safety and efficacy in chemoprevention. Previous chemoprevention randomised controlled trials (RCTs) did not individually show benefit of HCQ against COVID-19 and, although meta-analysis did suggest clinical benefit, guidelines recommend against its use.
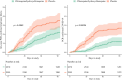
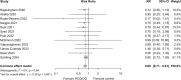
Methods and findings: Healthy adult participants from the healthcare setting, and later from the community, were enrolled in 26 centres in 11 countries to a double-blind, placebo-controlled, randomised trial of COVID-19 chemoprevention. HCQ was evaluated in Europe and Africa, and chloroquine (CQ) was evaluated in Asia, (both base equivalent of 155 mg once daily). The primary endpoint was symptomatic COVID-19, confirmed by PCR or seroconversion during the 3-month follow-up period. The secondary and tertiary endpoints were: asymptomatic laboratory-confirmed Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection; severity of COVID-19 symptoms; all-cause PCR-confirmed symptomatic acute respiratory illness (including SARS-CoV-2 infection); participant reported number of workdays lost; genetic and baseline biochemical markers associated with symptomatic COVID-19, respiratory illness and disease severity (not reported here); and health economic analyses of HCQ and CQ prophylaxis on costs and quality of life measures (not reported here). The primary and safety analyses were conducted in the intention-to-treat (ITT) population. Recruitment of 40,000 (20,000 HCQ arm, 20,000 CQ arm) participants was planned but was not possible because of protracted delays resulting from controversies over efficacy and adverse events with HCQ use, vaccine rollout in some countries, and other factors. Between 29 April 2020 and 10 March 2022, 4,652 participants (46% females) were enrolled (HCQ/CQ n = 2,320; placebo n = 2,332). The median (IQR) age was 29 (23 to 39) years. SARS-CoV-2 infections (symptomatic and asymptomatic) occurred in 1,071 (23%) participants. For the primary endpoint the incidence of symptomatic COVID-19 was 240/2,320 in the HCQ/CQ versus 284/2,332 in the placebo arms (risk ratio (RR) 0.85 [95% confidence interval, 0.72 to 1.00; p = 0.05]). For the secondary and tertiary outcomes asymptomatic SARS-CoV-2 infections occurred in 11.5% of HCQ/CQ recipients and 12.0% of placebo recipients: RR: 0.96 (95% CI, 0.82 to 1.12; p = 0.6). There were no differences in the severity of symptoms between the groups and no severe illnesses. HCQ/CQ chemoprevention was associated with fewer PCR-confirmed all-cause respiratory infections (predominantly SARS-CoV-2): RR 0.61 (95% CI, 0.42 to 0.88; p = 0.009) and fewer days lost to work because of illness: 104 days per 1,000 participants over 90 days (95% CI, 12 to 199 days; p < 0.001). The prespecified meta-analysis of all published pre-exposure RCTs indicates that HCQ/CQ prophylaxis provided a moderate protective benefit against symptomatic COVID-19: RR 0.80 (95% CI, 0.71 to 0.91). Both drugs were well tolerated with no drug-related serious adverse events (SAEs). Study limitations include the smaller than planned study size, the relatively low number of PCR-confirmed infections, and the lower comparative accuracy of serology endpoints (in particular, the adapted dried blood spot method) compared to the PCR endpoint. The COPCOV trial was registered with ClinicalTrials.gov; number NCT04303507.
Interpretation: In this large placebo-controlled, double-blind randomised trial, HCQ and CQ were safe and well tolerated in COVID-19 chemoprevention, and there was evidence of moderate protective benefit in a meta-analysis including this trial and similar RCTs.
Trial registration: ClinicalTrials.gov NCT04303507; ISRCTN Registry ISRCTN10207947.
Copyright: © 2024 Schilling et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
NJW and LvS are members of the PLOS Medicine Editorial Board. The rest of the authors have declared that no competing interests exist.
Figures

References
-
- World Health Organization WHO Coronavirus (COVID-19) Dashboard. Available from: https://covid19.who.int/. Last accessed 2023 Oct 25.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

