Development of a Lipid-encapsulated TGFβRI-siRNA Drug for Liver Fibrosis Induced by Schistosoma mansoni
- PMID: 39264964
- PMCID: PMC11421824
- DOI: 10.1371/journal.pntd.0012502
Development of a Lipid-encapsulated TGFβRI-siRNA Drug for Liver Fibrosis Induced by Schistosoma mansoni
Abstract
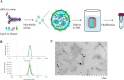
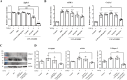
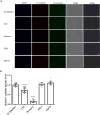
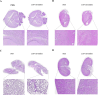
Schistosoma mansoni infection leads to chronic schistosomiasis and severe hepatic fibrosis. We designed a liver-targeted lipid nanoparticle (LNP) carrying siRNA against type I TGF-β receptor (TGFβRI) mRNA to treat schistosomiasis-induced liver fibrosis in BALB/c mice. Knockdown of TGFβRI by LNP-siTGFβRI reduced LX-2 cell activation in vitro and alleviated liver fibrosis in S. mansoni-infected mice. αSMA and Col1a1 fibrotic markers in the liver tissues of infected mice were significantly suppressed in the treatment groups. In the serum of the LNP-siTGFβRI-treated groups, cytokines IFNγ, IL-1α, IL-6, IL-12, RANTES (CCL5), and TNFα increased, while GM-CSF, IL-2, IL-4, IL-10, IL-13, and KC (CXCL1) decreased compared to the control. Cell proportions were significantly altered in S. mansoni-infected mice, with increased CD56d NK cells and decreased CD19+ B cells and CD4+ T cells compared to naïve mice. Following LNP-siTGFβRI treatment, CD56d NK cells were downregulated, while B and memory Th cell populations were upregulated. The density of fibrotic regions significantly decreased with LNP-siTGFβRI treatment in a dose-dependent manner, and no systemic toxicity was observed in the major organs. This targeted siRNA delivery strategy effectively reduced granulomatous lesions in schistosomiasis-induced liver fibrosis without detectable side effects.
Copyright: © 2024 Chen et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

