Nickel-Iron Layered Double Hydroxides/Nickel Sulfide Heterostructured Electrocatalysts on Surface-Modified Ti Foam for the Oxygen Evolution Reaction
- PMID: 39265050
- PMCID: PMC11440454
- DOI: 10.1021/acsami.4c08215
Nickel-Iron Layered Double Hydroxides/Nickel Sulfide Heterostructured Electrocatalysts on Surface-Modified Ti Foam for the Oxygen Evolution Reaction
Abstract
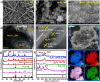
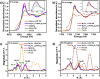
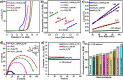
Electrochemical approaches for generating hydrogen from water splitting can be more promising if the challenges in the anodic oxygen evolution reaction (OER) can be harnessed. The interface heterostructure materials offer strong electronic coupling and appropriate charge transport at the interface regions, promoting accessible active sites to prompt kinetics and optimize the adsorption-desorption of active species. Herein, we have designed an efficient multi-interface-engineered Ni3Fe1 LDH/Ni3S2/TW heterostructure on in situ generated titanate web layers from the titanium foam. The synergistic effects of the multi-interface heterostructure caused the exposure of rich interfacial electronic coupling, fast reaction kinetics, and enhanced accessible site activity and site populations. The as-prepared electrocatalyst demonstrates outstanding OER activity, demanding a low overpotential of 220 mV at a high current density of 100 mA cm-2. Similarly, the designed Ni3Fe1 LDH/Ni3S2/TW electrocatalyst exhibits a low Tafel slope of 43.2 mV dec-1 and excellent stability for 100 h of operation, suggesting rapid kinetics and good structural stability. Also, the electrocatalyst shows a low overpotential of 260 mV at 100 mA cm-2 for HER activity. Moreover, the integrated electrocatalyst exhibits an incredible OER activity in simulated seawater with an overpotential of 370 mV at 100 mA cm-2 and stability for 100 h of operation, indicating good OER selectivity. This work might benefit further fabricating effective and stable self-sustained electrocatalysts for water splitting in large-scale applications.
Keywords: heterostructure; multi-interface; stability; synergistic effect; water splitting.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Fauzi A.; Geng S.; Tian F.; Liu Y.; Li H.; Yu Y.; Li J.; Yang W. NiFe-LDH@Ni3S2 Supported on Nickel Foam as Highly Active Electrocatalysts for Oxygen Evolution Reaction. Int. J. Hydrog. Energy 2023, 48 (4), 1370–1379. 10.1016/j.ijhydene.2022.09.305. - DOI
-
- Adam D. B.; Tsai M.-C.; Awoke Y. A.; Huang W.-H.; Yang Y.-W.; Pao C.-W.; Su W.-N.; Hwang B. J. Iodide Oxidation Reaction Catalyzed by Ruthenium–Tin Surface Alloy Oxide for Efficient Production of Hydrogen and Iodine Simultaneously. ACS Sustain. Chem. Eng. 2021, 9 (26), 8803–8812. 10.1021/acssuschemeng.1c01867. - DOI
-
- Wu Q.; Gao Q.; Sun L.; Guo H.; Tai X.; Li D.; Liu L.; Ling C.; Sun X. Facilitating Active Species by Decorating CeO2 on Ni3S2 Nanosheets for Efficient Water Oxidation Electrocatalysis. Chin. J. Catal. 2021, 42 (3), 482–489. 10.1016/S1872-2067(20)63663-4. - DOI
LinkOut - more resources
Full Text Sources
Research Materials

