Spns1 is an iron transporter essential for megalin-dependent endocytosis
- PMID: 39265081
- PMCID: PMC11563593
- DOI: 10.1152/ajprenal.00172.2024
Spns1 is an iron transporter essential for megalin-dependent endocytosis
Abstract
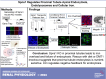
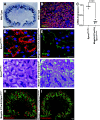
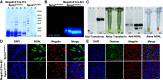
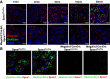
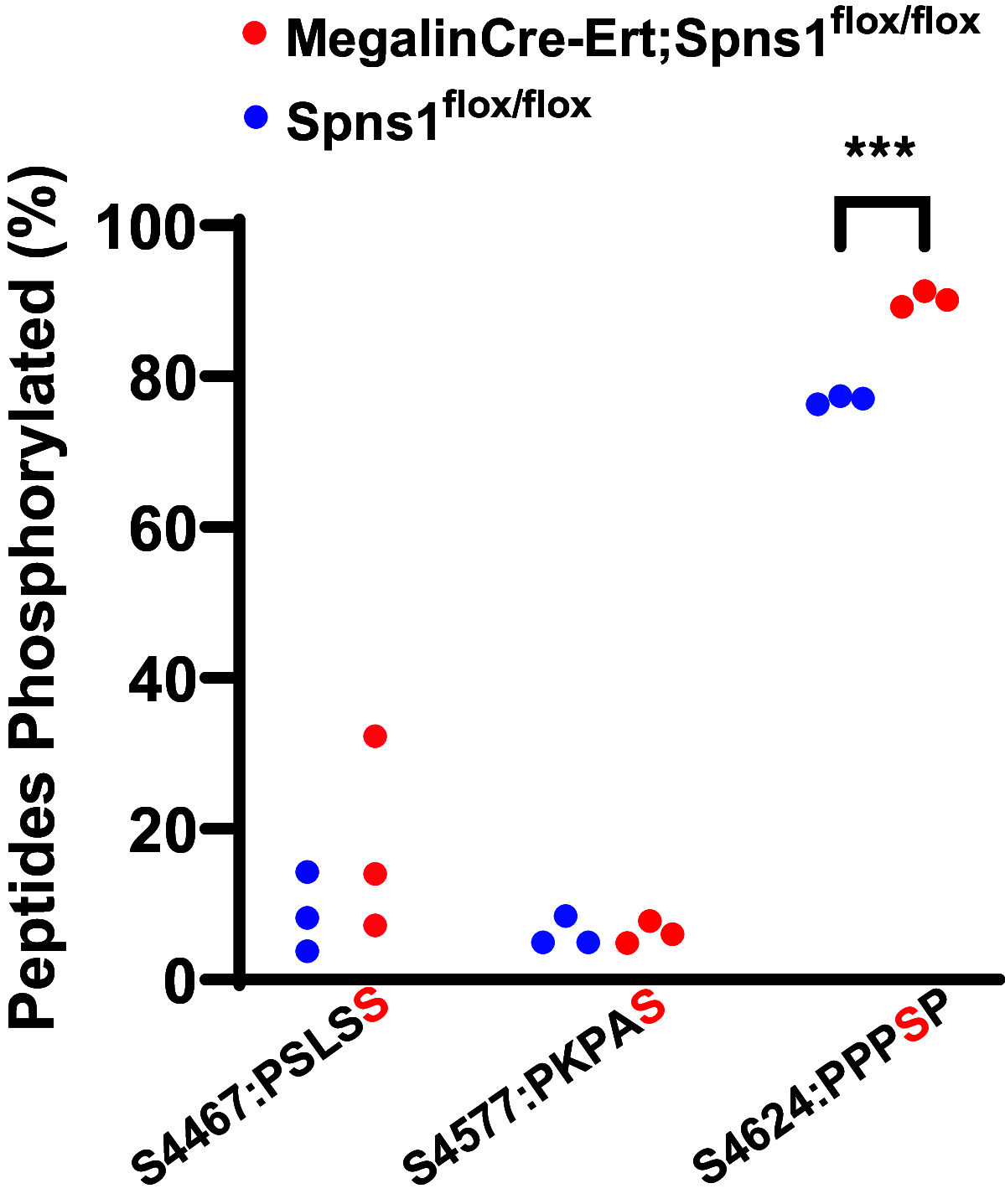
Proximal tubule endocytosis is essential to produce protein-free urine as well as to regulate system-wide metabolic pathways, such as the activation of Vitamin D. We have determined that the proximal tubule expresses an endolysosomal membrane protein, protein spinster homolog1 (Spns1), which engenders a novel iron conductance that is indispensable during embryonic development. Conditional knockout of Spns1 with a novel Cre-LoxP construct specific to megalin-expressing cells led to the arrest of megalin receptor-mediated endocytosis as well as dextran pinocytosis in proximal tubules. The endocytic defect was accompanied by changes in megalin phosphorylation as well as enlargement of lysosomes, confirming previous findings in Drosophila and Zebrafish. The endocytic defect was also accompanied by iron overload in proximal tubules. Remarkably, iron levels regulated the Spns1 phenotypes because feeding an iron-deficient diet or mating Spns1 knockout with divalent metal transporter1 knockout rescued the phenotypes. Conversely, iron-loading wild-type mice reproduced the endocytic defect. These data demonstrate a reversible, negative feedback for apical endocytosis and raise the possibility that regulation of endocytosis, pinocytosis, megalin activation, and organellar size and function is nutrient-responsive.NEW & NOTEWORTHY Spns1 mediates a novel iron conductance essential during embryogenesis. Spns1 knockout leads to endocytic and lysosomal defects, accompanied by iron overload in the kidney. Reversal of iron overload by restricting dietary iron or by concurrent knockout of the iron transporter, DMT1 rescued the endocytic and organellar defects and reverted markers of iron overload. These data suggest feedback between iron and proximal tubule endocytosis.
Keywords: Spns1; endocytosis; iron; megalin; proteinuria.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures


References
MeSH terms
Substances
Grants and funding
- K08-DK132511/HHS | NIH | National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)
- 18840098/American Heart Association (AHA)
- K01 DK135917/DK/NIDDK NIH HHS/United States
- P30CA013696/HHS | NIH | National Cancer Institute (NCI)
- R01 DK124667/DK/NIDDK NIH HHS/United States
- DK124667/HHS | NIH | National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)
- K25-DK128563/HHS | NIH | National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)
- U54-DK104309/HHS | NIH | National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)
- K08 DK132511/DK/NIDDK NIH HHS/United States
- 1S10-RR027990/HHS | NIH | National Center for Research Resources (NCRR)
- Alpha Institute of Natural Medicine/Tongji University
- TL1 DK136048/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

