Lipocalin-2 aggravates blood-brain barrier dysfunction after intravenous thrombolysis by promoting endothelial cell ferroptosis via regulating the HMGB1/Nrf2/HO-1 pathway
- PMID: 39265498
- PMCID: PMC11415874
- DOI: 10.1016/j.redox.2024.103342
Lipocalin-2 aggravates blood-brain barrier dysfunction after intravenous thrombolysis by promoting endothelial cell ferroptosis via regulating the HMGB1/Nrf2/HO-1 pathway
Abstract
Background: Disruption of the blood-brain barrier (BBB) is a major contributor to hemorrhagic transformation (HT) in patients with acute ischemic stroke (AIS) following intravenous thrombolysis (IVT). However, the clinical therapies aimed at BBB protection after IVT remain limited.
Methods: One hundred patients with AIS who underwent IVT were enrolled (42 with HT and 58 without HT 24 h after IVT). Based on the cytokine chip, the serum levels of several AIS-related proteins, including LCN2, ferritin, matrix metalloproteinase-3, vascular endothelial-derived growth factor, and X-linked inhibitor of apoptosis, were detected upon admission, and their associations with HT were analyzed. After finding that LCN2 was related to HT in patients with IVT, we clarified whether the modulation of LCN2 influenced BBB dysfunction and HT after thrombolysis and investigated the potential mechanism.
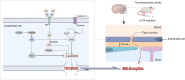
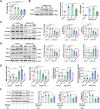
Results: In patients with AIS following IVT, logistic regression analysis showed that baseline serum LCN2 (p = 0.023) and ferritin (p = 0.046) levels were independently associated with HT. A positive correlation between serum LCN2 and ferritin levels was identified in patients with HT. In experimental studies, recombinant LCN2 (rLCN2) significantly aggravated BBB dysfunction and HT in the thromboembolic stroke rats after thrombolysis, whereas LCN2 inhibition by ZINC006440089 exerted opposite effects. Further mechanistic studies showed that, LCN2 promoted endothelial cell ferroptosis, accompanied by the induction of high mobility group box 1 (HMGB1) and the inhibition of nuclear translocation of nuclear factor E2-related factor 2 (Nrf2) and heme oxygenase-1 (HO-1) proteins. Ferroptosis inhibitor ferrostatin-1 (fer-1) significantly restricted the LCN2-mediated BBB disruption. Transfection of LCN2 and HMGB1 siRNA inhibited the endothelial cell ferroptosis, and this effects was reversed by Nrf2 siRNA.
Conclusion: LCN2 aggravated BBB disruption after thrombolysis by promoting endothelial cell ferroptosis via regulating the HMGB1/Nrf2/HO-1 pathway, this may provide a promising therapeutic target for the prevention of HT after IVT.
Keywords: Blood-brain barrier; Ferroptosis; Hemorrhagic transformation; High mobility group box 1; Intravenous thrombolysis; Lipocalin-2.
Copyright © 2024. Published by Elsevier B.V.
Conflict of interest statement
Declaration of competing interest None.
Figures







References
-
- Powers W.J., Rabinstein A.A., Ackerson T., Adeoye O.M., Bambakidis N.C., Becker K., Biller J., Brown M., Demaerschalk B.M., Hoh B., Jauch E.C., Kidwell C.S., Leslie-Mazwi T.M., Ovbiagele B., Scott P.A., Sheth K.N., Southerland A.M., Summers D.V., Tirschwell D.L. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke. 2019;50:e344–e418. - PubMed
-
- Shi K., Zou M., Jia D.M., Shi S., Yang X., Liu Q., Dong J.F., Sheth K.N., Wang X., Shi F.D. tPA mobilizes immune cells that exacerbate hemorrhagic transformation in stroke. Circ. Res. 2021;128:62–75. - PubMed
-
- Chen H., Guan B., Chen X., Chen X., Li C., Qiu J., Yang D., Liu K.J., Qi S., Shen J. Baicalin attenuates blood-brain barrier disruption and hemorrhagic transformation and improves neurological outcome in ischemic stroke rats with delayed t-PA treatment: involvement of ONOO(-)-MMP-9 pathway. Transl Stroke Res. 2018;9:515–529. - PubMed
-
- Ozkul-Wermester O., Guegan-Massardier E., Triquenot A., Borden A., Perot G., Gérardin E. Increased blood-brain barrier permeability on perfusion computed tomography predicts hemorrhagic transformation in acute ischemic stroke. Eur. Neurol. 2014;72:45–53. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

