Spheroid-Hydrogel-Integrated Biomimetic System: A New Frontier in Advanced Three-Dimensional Cell Culture Technology
- PMID: 39265553
- PMCID: PMC11965833
- DOI: 10.1159/000541416
Spheroid-Hydrogel-Integrated Biomimetic System: A New Frontier in Advanced Three-Dimensional Cell Culture Technology
Abstract
Background: Despite significant advances in three-dimensional (3D) cell culture technologies, creating accurate in vitro models that faithfully recapitulate complex in vivo environments remains a major challenge in biomedical research. Traditional culture methods often fail to simultaneously facilitate critical cell-cell and cell-extracellular matrix (ECM) interactions while providing control over mechanical and biochemical properties.
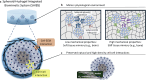
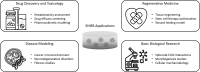
Summary: This review introduces the spheroid-hydrogel-integrated biomimetic system (SHIBS), a groundbreaking approach that synergistically combines spheroid culture with tailored hydrogel technologies. SHIBS uniquely bridges the gap between traditional culture methods and physiological conditions by offering unprecedented control over both cellular interactions and environmental properties. We explore how SHIBS is revolutionizing fields ranging from drug discovery and disease modeling to regenerative medicine and basic biological research. The review discusses current challenges in SHIBS technology, including reproducibility, scalability, and high-resolution imaging, and outlines ongoing research addressing these issues. Furthermore, we envision the future evolution of SHIBS into more sophisticated organoid-hydrogel-integrated biomimetic systems and its integration with cutting-edge technologies such as microfluidics, 3D bioprinting, and artificial intelligence.
Key messages: SHIBS represents a paradigm shift in 3D cell culture technology, offering a unique solution to recreate complex in vivo environments. Its potential to accelerate the development of personalized therapies across various biomedical fields is significant. While challenges persist, the ongoing advancements in SHIBS technology promise to overcome current limitations, paving the way for more accurate and reliable in vitro models. The future integration of SHIBS with emerging technologies may revolutionize biomimetic modeling, potentially reducing the need for animal testing and expediting drug discovery processes. This comprehensive review provides researchers and clinicians with a holistic understanding of SHIBS technology, its current capabilities, and its future prospects in advancing biomedical research and therapeutic innovations.
Background: Despite significant advances in three-dimensional (3D) cell culture technologies, creating accurate in vitro models that faithfully recapitulate complex in vivo environments remains a major challenge in biomedical research. Traditional culture methods often fail to simultaneously facilitate critical cell-cell and cell-extracellular matrix (ECM) interactions while providing control over mechanical and biochemical properties.
Summary: This review introduces the spheroid-hydrogel-integrated biomimetic system (SHIBS), a groundbreaking approach that synergistically combines spheroid culture with tailored hydrogel technologies. SHIBS uniquely bridges the gap between traditional culture methods and physiological conditions by offering unprecedented control over both cellular interactions and environmental properties. We explore how SHIBS is revolutionizing fields ranging from drug discovery and disease modeling to regenerative medicine and basic biological research. The review discusses current challenges in SHIBS technology, including reproducibility, scalability, and high-resolution imaging, and outlines ongoing research addressing these issues. Furthermore, we envision the future evolution of SHIBS into more sophisticated organoid-hydrogel-integrated biomimetic systems and its integration with cutting-edge technologies such as microfluidics, 3D bioprinting, and artificial intelligence.
Key messages: SHIBS represents a paradigm shift in 3D cell culture technology, offering a unique solution to recreate complex in vivo environments. Its potential to accelerate the development of personalized therapies across various biomedical fields is significant. While challenges persist, the ongoing advancements in SHIBS technology promise to overcome current limitations, paving the way for more accurate and reliable in vitro models. The future integration of SHIBS with emerging technologies may revolutionize biomimetic modeling, potentially reducing the need for animal testing and expediting drug discovery processes. This comprehensive review provides researchers and clinicians with a holistic understanding of SHIBS technology, its current capabilities, and its future prospects in advancing biomedical research and therapeutic innovations.
Keywords: Biomimetic systems; Drug screening; Spheroid-hydrogel interaction; Three-dimensional cell culture; Tissue engineering.
© 2024 The Author(s). Published by S. Karger AG, Basel.
Conflict of interest statement
The author has no conflicts of interest to declare.
Figures

Similar articles
-
Uniform sized cancer spheroids production using hydrogel-based droplet microfluidics: a review.Biomed Microdevices. 2024 May 29;26(2):26. doi: 10.1007/s10544-024-00712-3. Biomed Microdevices. 2024. PMID: 38806765 Free PMC article. Review.
-
iPSC-derived and Patient-Derived Organoids: Applications and challenges in scalability and reproducibility as pre-clinical models.Curr Res Toxicol. 2024 Oct 2;7:100197. doi: 10.1016/j.crtox.2024.100197. eCollection 2024. Curr Res Toxicol. 2024. PMID: 40276485 Free PMC article.
-
Integrating microfluidics, hydrogels, and 3D bioprinting for personalized vessel-on-a-chip platforms.Biomater Sci. 2025 Feb 25;13(5):1131-1160. doi: 10.1039/d4bm01354a. Biomater Sci. 2025. PMID: 39834160 Review.
-
Dynamic Culture of Bioprinted Liver Tumor Spheroids in a Pillar/Perfusion Plate for Predictive Screening of Anticancer Drugs.Biotechnol Bioeng. 2025 Apr;122(4):995-1009. doi: 10.1002/bit.28924. Epub 2025 Jan 16. Biotechnol Bioeng. 2025. PMID: 39821523
-
Microfluidic-assisted engineering of hydrogels with microscale complexity.Acta Biomater. 2025 Jun 1;199:1-17. doi: 10.1016/j.actbio.2025.05.023. Epub 2025 May 9. Acta Biomater. 2025. PMID: 40349902 Review.
Cited by
-
Exploring Experimental Models of Colorectal Cancer: A Critical Appraisal from 2D Cell Systems to Organoids, Humanized Mouse Avatars, Organ-on-Chip, CRISPR Engineering, and AI-Driven Platforms-Challenges and Opportunities for Translational Precision Oncology.Cancers (Basel). 2025 Jun 26;17(13):2163. doi: 10.3390/cancers17132163. Cancers (Basel). 2025. PMID: 40647462 Free PMC article. Review.
References
-
- Pampaloni F, Reynaud EG, Stelzer EHK. The third dimension bridges the gap between cell culture and live tissue. Nat Rev Mol Cell Biol. 2007;8(10):839–45. - PubMed
-
- Abbott A. Cell culture: biology's new dimension. Nature. 2003;424(6951):870–2. - PubMed
-
- Lee S-Y, Koo I-S, Hwang HJ, Lee DW. In Vitro three-dimensional (3D) cell culture tools for spheroid and organoid models. SLAS Discov. 2023;28(4):119–37. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

