Structural organization of pyruvate: ferredoxin oxidoreductase from the methanogenic archaeon Methanosarcina acetivorans
- PMID: 39265575
- PMCID: PMC11956543
- DOI: 10.1016/j.str.2024.08.011
Structural organization of pyruvate: ferredoxin oxidoreductase from the methanogenic archaeon Methanosarcina acetivorans
Abstract
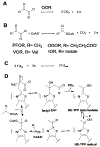
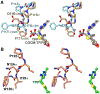
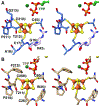
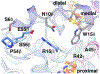
Enzymes of the 2-oxoacid:ferredoxin oxidoreductase (OFOR) superfamily catalyze the reversible oxidation of 2-oxoacids to acyl-coenzyme A esters and carbon dioxide (CO2)using ferredoxin or flavodoxin as the redox partner. Although members of the family share primary sequence identity, a variety of domain and subunit arrangements are known. Here, we characterize the structure of a four-subunit family member: the pyruvate:ferredoxin oxidoreductase (PFOR) from the methane producing archaeon Methanosarcina acetivorans (MaPFOR). The 1.92 Å resolution crystal structure of MaPFOR shows a protein fold like those of single- or two-subunit PFORs that function in 2-oxoacid oxidation, including the location of the requisite thiamine pyrophosphate (TPP), and three [4Fe-4S] clusters. Of note, MaPFOR typically functions in the CO2 reductive direction, and structural comparisons to the pyruvate oxidizing PFORs show subtle differences in several regions of catalytical relevance. These studies provide a framework that may shed light on the biochemical mechanisms used to facilitate reductive pyruvate synthesis.
Copyright © 2024 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures



References
-
- Charon MH, Volbeda A, Chabriere E, Pieulle L, and Fontecilla-Camps JC (1999). Structure and electron transfer mechanism of pyruvate:ferredoxin oxidoreductase. Curr Opin Struct Biol 9, 663–669. - PubMed
-
- Ragsdale SW (2003). Pyruvate ferredoxin oxidoreductase and its radical intermediate. Chem Rev 103, 2333–2346. - PubMed
-
- Yun NR, Arai H, Ishii M, and Igarashi Y (2001). The genes for anabolic 2-oxoglutarate: ferredoxin oxidoreductase from Hydrogenobacter thermophilus TK-6. Biochem Biophys Res Commun 282, 589–594. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

