Mechanical Thrombectomy Versus Intravenous Thrombolysis in Distal Medium Vessel Acute Ischemic Stroke: A Multinational Multicenter Propensity Score-Matched Study
- PMID: 39266014
- PMCID: PMC11471362
- DOI: 10.5853/jos.2024.01389
Mechanical Thrombectomy Versus Intravenous Thrombolysis in Distal Medium Vessel Acute Ischemic Stroke: A Multinational Multicenter Propensity Score-Matched Study
Abstract
Background and purpose: The management of acute ischemic stroke (AIS) due to distal medium vessel occlusion (DMVO) remains uncertain, particularly in comparing the effectiveness of intravenous thrombolysis (IVT) plus mechanical thrombectomy (MT) versus IVT alone. This study aimed to evaluate the safety and efficacy in DMVO patients treated with either MT-IVT or IVT alone.
Methods: This multinational study analyzed data from 37 centers across North America, Asia, and Europe. Patients with AIS due to DMVO were included, with data collected from September 2017 to July 2023. The primary outcome was functional independence, with secondary outcomes including mortality and safety measures such as types of intracerebral hemorrhage.
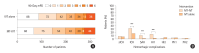
Results: The study involved 1,057 patients before matching, and 640 patients post-matching. Functional outcomes at 90 days showed no significant difference between groups in achieving good functional recovery (modified Rankin Scale 0-1 and 0-2), with adjusted odds ratios (OR) of 1.21 (95% confidence interval [CI] 0.81 to 1.79; P=0.35) and 1.00 (95% CI 0.66 to 1.51; P>0.99), respectively. Mortality rates at 90 days were similar between the two groups (OR 0.75, 95% CI 0.44 to 1.29; P=0.30). The incidence of symptomatic intracerebral hemorrhage was comparable, but any type of intracranial hemorrhage was significantly higher in the MT-IVT group (OR 0.43, 95% CI 0.29 to 0.63; P<0.001).
Conclusion: The results of this study indicate that while MT-IVT and IVT alone show similar functional and mortality outcomes in DMVO patients, MT-IVT presents a higher risk of hemorrhagic complications, thus MT-IVT may not routinely offer additional benefits over IVT alone for all DMVO stroke patients. Further prospective randomized trials are needed to identify patient subgroups most likely to benefit from MT-IVT treatment in DMVO.
Keywords: Distal medium vessel occlusions; Mechanical thrombectomy; Stroke.
Conflict of interest statement
Dr. Regenhardt serves on a DSMB for a trial sponsored by Rapid Medical, serves as site PI for studies sponsored by Penumbra and Microvention, and receives stroke research grant funding from the National Institutes of Health, Society of Vascular and Interventional Neurology, and Heitman Stroke Foundation. Dr. Guenego reports consultancy for Rapid Medical and Phenox, not directly related to the present work. Dr. Clarençon reports conflicts of interest with Medtronic, Balt Extrusion (consultant), ClinSearch (core lab), Penumbra, Stryker (payment for reading) and Artedrone (Board); all not directly related to the present work. Dr. Henninger received support from CDMRP/DoD W81XWH-19-PRARP-RPA and NINDS NS131756, during the conduct of the study. Dr. Liebeskind is consultant as Imaging Core Lab to Cerenovus, Genentech, Medtronic, Stryker, Rapid Medical. Dr. Yeo reports Advisory work for AstraZeneca, Substantial support from NMRC Singapore and is a medical advisor for See-mode, Cortiro and Sunbird Bio, with equity in Ceroflo. All unrelated to the present work. Dr. Griessenauer reports a proctoring agreement with Medtronic and research funding by Penumbra. Dr. Marnat reports conflicts of interest with Microvention Europe, Stryker Neurovascular, Balt (consulting), Medtronic, Johnson & Johnson and Phenox (paid lectures), all not directly related to the present work. Dr. Puri is a consultant for Medtronic Neurovascular, Stryker NeurovascularBalt, Q’Apel Medical, Cerenovus, Microvention, Imperative Care, Agile, Merit, CereVasc and Arsenal Medical, he received research grants from NIH, Microvention, Cerenovus, Medtronic Neurovascular and Stryker Neurovascular, and holds stocks in InNeuroCo, Agile, Perfuze, Galaxy and NTI. Dr. Tjoumakaris is a consultant for Medtronic and Microvention (funds paid to institution, not personally). Dr. Jabbour is a consultant for Medtronic, Microvention and Cerus. All remaining authors have declared no conflicts of interest.
Figures


References
-
- Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372:11–20. - PubMed
-
- Jovin TG, Chamorro A, Cobo E, de Miquel MA, Molina CA, Rovira A, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372:2296–2306. - PubMed
-
- Saver JL, Goyal M, Bonafe A, Diener HC, Levy EI, Pereira VM, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 2015;372:2285–2295. - PubMed
-
- Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372:1019–1030. - PubMed
-
- Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387:1723–1731. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

