Crosstalk between bone metastatic cancer cells and sensory nerves in bone metastatic progression
- PMID: 39266299
- PMCID: PMC11393574
- DOI: 10.26508/lsa.202302041
Crosstalk between bone metastatic cancer cells and sensory nerves in bone metastatic progression
Abstract
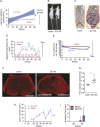
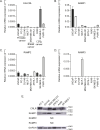
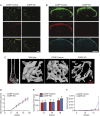
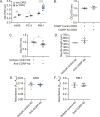
Although the role of peripheral nerves in cancer progression has been appreciated, little is known regarding cancer/sensory nerve crosstalk and its contribution to bone metastasis and associated pain. In this study, we revealed that the cancer/sensory nerve crosstalk plays a crucial role in bone metastatic progression. We found that (i) periosteal sensory nerves expressing calcitonin gene-related peptide (CGRP) are enriched in mice with bone metastasis; (ii) cancer patients with bone metastasis have elevated CGRP serum levels; (iii) bone metastatic patient tumor samples express elevated calcitonin receptor-like receptor (CRLR, a CGRP receptor component); (iv) higher CRLR levels in cancer patients are negatively correlated with recurrence-free survival; (v) CGRP induces cancer cell proliferation through the CRLR/p38/HSP27 pathway; and (vi) blocking sensory neuron-derived CGRP reduces cancer cell proliferation in vitro and bone metastatic progression in vivo. This suggests that CGRP-expressing sensory nerves are involved in bone metastatic progression and that the CGRP/CRLR axis may serve as a potential therapeutic target for bone metastasis.
© 2024 Park et al.
Conflict of interest statement
Part of the study was funded by Teva Pharmaceuticals (Yusuke Shiozawa). KD Mackenzie and JR Stratton were employed by Teva Pharmaceuticals. No conflict of interest exists for the remaining authors.
Figures













References
-
- Badrising SK, van der Noort V, Hamberg P, Coenen JL, Aarts MJ, van Oort IM, van den Eertwegh AJ, Los M, van den Berg HP, Gelderblom H, et al. (2016) Enzalutamide as a fourth- or fifth-line treatment option for metastatic castration-resistant prostate cancer. Oncology 91: 267–273. 10.1159/000448219 - DOI - PubMed
-
- Benyamin R, Trescot AM, Datta S, Buenaventura R, Adlaka R, Sehgal N, Glaser SE, Vallejo R (2008) Opioid complications and side effects. Pain Physician 11: S105–S120. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
