Synaptotagmin 4 Supports Spontaneous Axon Sprouting after Spinal Cord Injury
- PMID: 39266302
- PMCID: PMC11502230
- DOI: 10.1523/JNEUROSCI.1593-23.2024
Synaptotagmin 4 Supports Spontaneous Axon Sprouting after Spinal Cord Injury
Abstract
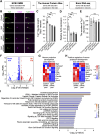
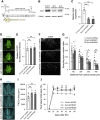
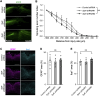
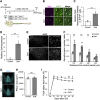
Injuries to the central nervous system (CNS) can cause severe neurological deficits. Axonal regrowth is a fundamental process for the reconstruction of compensatory neuronal networks after injury; however, it is extremely limited in the adult mammalian CNS. In this study, we conducted a loss-of-function genetic screen in cortical neurons, combined with a Web resource-based phenotypic screen, and identified synaptotagmin 4 (Syt4) as a novel regulator of axon elongation. Silencing Syt4 in primary cultured cortical neurons inhibits neurite elongation, with changes in gene expression involved in signaling pathways related to neuronal development. In a spinal cord injury model, inhibition of Syt4 expression in cortical neurons prevented axonal sprouting of the corticospinal tract, as well as neurological recovery after injury. These results provide a novel therapeutic approach to CNS injury by modulating Syt4 function.
Keywords: axon regeneration; corticospinal tract; spinal cord.
Copyright © 2024 Higuchi et al.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
