Hybrid lipid-AuNP clusters as highly efficient SERS substrates for biomedical applications
- PMID: 39266504
- PMCID: PMC11392932
- DOI: 10.1038/s41467-024-52205-9
Hybrid lipid-AuNP clusters as highly efficient SERS substrates for biomedical applications
Abstract
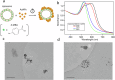
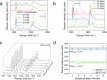
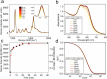
Although Surface Enhanced Raman Scattering (SERS) is widely applied for ultrasensitive diagnostics and imaging, its potential is largely limited by the difficult preparation of SERS tags, typically metallic nanoparticles (NPs) functionalized with Raman-active molecules (RRs), whose production often involves complex synthetic approaches, low colloidal stability and poor reproducibility. Here, we introduce LipoGold Tags, a simple platform where gold NPs (AuNPs) clusters form via self-assembly on lipid vesicle. RRs embedded in the lipid bilayer experience enhanced electromagnetic field, significantly increasing their Raman signals. We modulate RRs and lipid vesicle concentrations to achieve optimal SERS enhancement and we provide robust structural characterization. We further demonstrate the versatility of LipoGold Tags by functionalizing them with biomolecular probes, including antibodies. As proof of concept, we successfully detect intracellular GM1 alterations, distinguishing healthy donors from patients with infantile GM1 gangliosidosis, showcasing LipoGold Tags as advancement in SERS probes production.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Du, Z., Qi, Y., He, J., Zhong, D., Zhou, M. Recent advances in applications of nanoparticles in SERS in vivo imaging. WIREs Nanomed. Nanobiotechnol.13, 10.1002/wnan.1672 (2021). - PubMed
-
- Le Ru, C., Blackie, E., Meyer, M., & Etchegoin, P. G. Surface enhanced raman scattering enhancement factors: a comprehensive study. J. Phys. Chem. C.111, 13794–13803 (2007).10.1021/jp0687908 - DOI
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

