Engineered model of heart tissue repair for exploring fibrotic processes and therapeutic interventions
- PMID: 39266508
- PMCID: PMC11393355
- DOI: 10.1038/s41467-024-52221-9
Engineered model of heart tissue repair for exploring fibrotic processes and therapeutic interventions
Abstract
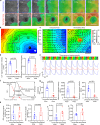
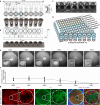
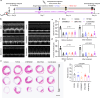
Advancements in human-engineered heart tissue have enhanced the understanding of cardiac cellular alteration. Nevertheless, a human model simulating pathological remodeling following myocardial infarction for therapeutic development remains essential. Here we develop an engineered model of myocardial repair that replicates the phased remodeling process, including hypoxic stress, fibrosis, and electrophysiological dysfunction. Transcriptomic analysis identifies nine critical signaling pathways related to cellular fate transitions, leading to the evaluation of seventeen modulators for their therapeutic potential in a mini-repair model. A scoring system quantitatively evaluates the restoration of abnormal electrophysiology, demonstrating that the phased combination of TGFβ inhibitor SB431542, Rho kinase inhibitor Y27632, and WNT activator CHIR99021 yields enhanced functional restoration compared to single factor treatments in both engineered and mouse myocardial infarction model. This engineered heart tissue repair model effectively captures the phased remodeling following myocardial infarction, providing a crucial platform for discovering therapeutic targets for ischemic heart disease.
© 2024. The Author(s).
Conflict of interest statement
For the development of the hEHT injury model and its application in drug screening, we have been granted a Chinese patent, with inventors Donghui Zhang, Pengcheng Yang, and Jixing Gong, and patent number ZL 2020 1 0620164. X. Besides that, the authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

