TFCP2L1 drives stemness and enhances their resistance to Sorafenib treatment by modulating the NANOG/STAT3 pathway in hepatocellular carcinoma
- PMID: 39266516
- PMCID: PMC11392926
- DOI: 10.1038/s41389-024-00534-1
TFCP2L1 drives stemness and enhances their resistance to Sorafenib treatment by modulating the NANOG/STAT3 pathway in hepatocellular carcinoma
Abstract
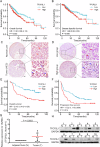
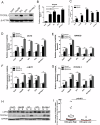
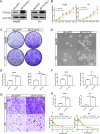
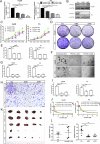
Hepatocellular carcinoma (HCC) is a prevalent and aggressive malignancy associated with high risks of recurrence and metastasis. Liver cancer stem cells (CSCs) are increasingly recognized as pivotal drivers of these processes. In our previous research, we demonstrated the involvement of TFCP2L1 in maintaining the pluripotency of embryonic stem cells. However, its relevance to liver CSCs remains unexplored. In this study, we report an inverse correlation between TFCP2L1 protein levels in HCC tissue and patient outcomes. The knockdown of TFCP2L1 significantly reduced HCC cell proliferation, invasion, metastasis, clonal formation, and sphere-forming capacity, while its overexpression enhanced these functions. In addition, experiments using a nude mouse model confirmed TFCP2L1's essential role in liver CSCs' function and tumorigenic potential. Mechanistically, we showed that TFCP2L1 promotes the stemness of CSCs by upregulating NANOG, which subsequently activates the JAK/STAT3 pathway, thereby contributing to HCC pathogenesis. Importantly, we identified a specific small molecule targeting TFCP2L1's active domain, which, in combination with Sorafenib, sensitizes hepatoma cells to treatment. Together, these findings underscore TFCP2L1's pathological significance in HCC progression, supporting its potential as a prognostic biomarker and therapeutic target in this disease.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
The transcription factor TFCP2L1 induces expression of distinct target genes and promotes self-renewal of mouse and human embryonic stem cells.J Biol Chem. 2019 Apr 12;294(15):6007-6016. doi: 10.1074/jbc.RA118.006341. Epub 2019 Feb 19. J Biol Chem. 2019. PMID: 30782842 Free PMC article.
-
ABI2-mediated MEOX2/KLF4-NANOG axis promotes liver cancer stem cell and drives tumour recurrence.Liver Int. 2022 Nov;42(11):2562-2576. doi: 10.1111/liv.15412. Epub 2022 Sep 6. Liver Int. 2022. PMID: 36017822 Free PMC article.
-
lncARSR promotes liver cancer stem cells expansion via STAT3 pathway.Gene. 2019 Mar 1;687:73-81. doi: 10.1016/j.gene.2018.10.087. Epub 2018 Oct 31. Gene. 2019. PMID: 30391438
-
Coexpression of gene Oct4 and Nanog initiates stem cell characteristics in hepatocellular carcinoma and promotes epithelial-mesenchymal transition through activation of Stat3/Snail signaling.J Hematol Oncol. 2015 Mar 11;8:23. doi: 10.1186/s13045-015-0119-3. J Hematol Oncol. 2015. PMID: 25879771 Free PMC article.
-
Nanog regulates self-renewal of cancer stem cells through the insulin-like growth factor pathway in human hepatocellular carcinoma.Hepatology. 2012 Sep;56(3):1004-14. doi: 10.1002/hep.25745. Epub 2012 Jul 12. Hepatology. 2012. PMID: 22473773
Cited by
-
N6-methyladenosine-modified long non-coding RNA KIF9-AS1 promotes stemness and sorafenib resistance in hepatocellular carcinoma by upregulating SHOX2 expression.World J Gastroenterol. 2024 Dec 28;30(48):5174-5190. doi: 10.3748/wjg.v30.i48.5174. World J Gastroenterol. 2024. PMID: 39735272 Free PMC article.
-
Therapeutic and Prognostic Relevance of Cancer Stem Cell Populations in Endometrial Cancer: A Narrative Review.Diagnostics (Basel). 2025 Jul 25;15(15):1872. doi: 10.3390/diagnostics15151872. Diagnostics (Basel). 2025. PMID: 40804837 Free PMC article. Review.
-
OSMR induces M2 polarization of glioblastoma associated macrophages through JAK/STAT3 signaling pathway.Front Oncol. 2025 Mar 14;15:1538649. doi: 10.3389/fonc.2025.1538649. eCollection 2025. Front Oncol. 2025. PMID: 40161370 Free PMC article.
-
Tfcp2l1 as a central integrator of hypoxia, dedifferentiation, and tumor progression.J Exp Clin Cancer Res. 2025 Aug 14;44(1):236. doi: 10.1186/s13046-025-03501-9. J Exp Clin Cancer Res. 2025. PMID: 40813991 Free PMC article. Review.
References
-
- Liao W, Coupland CAC, Innes H, Jepsen P, Matthews PC, Campbell C, et al. Disparities in care and outcomes for primary liver cancer in England during 2008-2018: a cohort study of 8.52 million primary care population using the QResearch database. EClinicalMedicine. 2023;59:101969. 10.1016/j.eclinm.2023.101969 - DOI - PMC - PubMed
-
- Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA: A Cancer J Clinicians. 2023;73:17–48. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

