Clearance and transport of amyloid β by peripheral monocytes correlate with Alzheimer's disease progression
- PMID: 39266542
- PMCID: PMC11393069
- DOI: 10.1038/s41467-024-52396-1
Clearance and transport of amyloid β by peripheral monocytes correlate with Alzheimer's disease progression
Abstract
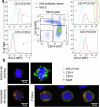
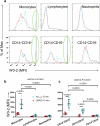
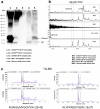
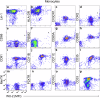
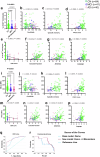
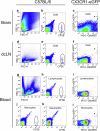
Impaired clearance of amyloid β (Aβ) in late-onset Alzheimer's disease (AD) affects disease progression. The role of peripheral monocytes in Aβ clearance from the central nervous system (CNS) is unclear. We use a flow cytometry assay to identify Aβ-binding monocytes in blood, validated by confocal microscopy, Western blotting, and mass spectrometry. Flow cytometry immunophenotyping and correlation with AD biomarkers are studied in 150 participants from the AIBL study. We also examine monocytes in human cerebrospinal fluid (CSF) and their migration in an APP/PS1 mouse model. The assay reveals macrophage-like Aβ-binding monocytes with high phagocytic potential in both the periphery and CNS. We find lower surface Aβ levels in mild cognitive impairment (MCI) and AD-dementia patients compared to cognitively unimpaired individuals. Monocyte infiltration from blood to CSF and migration from CNS to peripheral lymph nodes and blood are observed. Here we show that Aβ-binding monocytes may play a role in CNS Aβ clearance, suggesting their potential as a biomarker for AD diagnosis and monitoring.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
- 1048082/Department of Health | National Health and Medical Research Council (NHMRC)
- 1061419/Department of Health | National Health and Medical Research Council (NHMRC)
- 1120095/Department of Health | National Health and Medical Research Council (NHMRC)
- 110178/Department of Health | National Health and Medical Research Council (NHMRC)
- Operational Infrastructure Support Grant to the Florey Institute/State Government of Victoria (Victorian Government)
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

