A single-domain antibody targeting factor XII inhibits both thrombosis and inflammation
- PMID: 39266545
- PMCID: PMC11393108
- DOI: 10.1038/s41467-024-51745-4
A single-domain antibody targeting factor XII inhibits both thrombosis and inflammation
Abstract
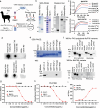
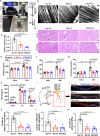
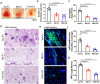
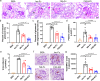
Factor XII (FXII) is the zymogen of the plasma protease FXIIa that activates the intrinsic coagulation pathway and the kallikrein kinin-system. The role of FXII in inflammation has been obscure. Here, we report a single-domain antibody (nanobody, Nb) fused to the Fc region of a human immunoglobulin (Nb-Fc) that recognizes FXII in a conformation-dependent manner and interferes with FXIIa formation. Nb-Fc treatment inhibited arterial thrombosis in male mice without affecting hemostasis. In a mouse model of extracorporeal membrane oxygenation (ECMO), FXII inhibition or knockout reduced thrombus deposition on oxygenator membranes and systemic microvascular thrombi. ECMO increased circulating levels of D-dimer, alkaline phosphatase, creatinine and TNF-α and triggered microvascular neutrophil adherence, platelet aggregation and their interaction, which were substantially attenuated by FXII blockade. Both Nb-Fc treatment and FXII knockout markedly ameliorated immune complex-induced local vasculitis and anti-neutrophil cytoplasmic antibody-induced systemic vasculitis, consistent with selectively suppressed neutrophil migration. In human blood microfluidic analysis, Nb-Fc treatment prevented collagen-induced fibrin deposition and neutrophil adhesion/activation. Thus, FXII is an important mediator of inflammatory responses in vasculitis and ECMO, and Nb-Fc provides a promising approach to alleviate thrombo-inflammatory disorders.
© 2024. The Author(s).
Conflict of interest statement
M.W. is a co-founder of NINGBO COMGEN BIOTECH CO., LTD. The remaining authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

