Engineering potent chimeric antigen receptor T cells by programming signaling during T-cell activation
- PMID: 39266656
- PMCID: PMC11392953
- DOI: 10.1038/s41598-024-72392-1
Engineering potent chimeric antigen receptor T cells by programming signaling during T-cell activation
Abstract
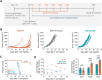
Programming cell signaling during T-cell activation represents a simple strategy for improving the potency of therapeutic T-cell products. Stim-R technology (Lyell Immunopharma) is a customizable, degradable synthetic cell biomimetic that emulates physiologic, cell-like presentation of signal molecules to control T-cell activation. A breadth of Stim-R formulations with different anti-CD3/anti-CD28 (αCD3/αCD28) antibody densities and stoichiometries were screened for their effects on multiple metrics of T-cell function. We identified an optimized formulation that produced receptor tyrosine kinase-like orphan receptor 1 (ROR1)-targeted chimeric antigen receptor (CAR) T cells with enhanced persistence and polyfunctionality in vitro, as assessed in repeat-stimulation assays, compared with a benchmark product generated using a conventional T-cell-activating reagent. In transcriptomic analyses, CAR T cells activated with Stim-R technology showed downregulation of exhaustion-associated gene sets and retained a unique subset of stem-like cells with effector-associated gene signatures following repeated exposure to tumor cells. Compared with the benchmark product, CAR T cells activated using the optimized Stim-R technology formulation exhibited higher peak expansion, prolonged persistence, and improved tumor control in a solid tumor xenograft model. Enhancing T-cell products with Stim-R technology during T-cell activation may help improve therapeutic efficacy against solid tumors.
© 2024. The Author(s).
Conflict of interest statement
A.S.C. is an inventor on a patent application related to the Stim-R technology (US patent 11555177B2). All authors were employees of Lyell Immunopharma at the time of study execution, and may additionally hold stock in Lyell Immunopharma, which has licensed intellectual property related to the Stim-R technology.
Figures




References
-
- Berdeja, J. G. et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): A phase 1b/2 open-label study. Lancet398, 314–324 (2021). 10.1016/S0140-6736(21)00933-8 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

