Barriers and opportunities in pancreatic cancer immunotherapy
- PMID: 39266715
- PMCID: PMC11393360
- DOI: 10.1038/s41698-024-00681-z
Barriers and opportunities in pancreatic cancer immunotherapy
Abstract
Pancreatic ductal adenocarcinoma (PDAC) presents a fatal clinical challenge characterized by a dismal 5-year overall survival rate, primarily due to the lack of early diagnosis and limited therapeutic efficacy. Immunotherapy, a proven success in multiple cancers, has yet to demonstrate significant benefits in PDAC. Recent studies have revealed the immunosuppressive characteristics of the PDAC tumor microenvironment (TME), including immune cells with suppressive properties, desmoplastic stroma, microbiome influences, and PDAC-specific signaling pathways. In this article, we review recent advances in understanding the immunosuppressive TME of PDAC, TME differences among various mouse models of pancreatic cancer, and the mechanisms underlying resistance to immunotherapeutic interventions. Furthermore, we discuss the potential of targeting cancer cell-intrinsic pathways and TME components to sensitize PDAC to immune therapies, providing insights into strategies and future perspectives to break through the barriers in improving pancreatic cancer treatment.
© 2024. The Author(s).
Conflict of interest statement
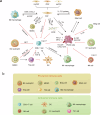
The authors declare no competing interests. Figure 2 was created with BioRender.com.
Figures


References
Publication types
Grants and funding
- R01CA269140/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- R01 CA166051/CA/NCI NIH HHS/United States
- R01 CA269140/CA/NCI NIH HHS/United States
- R01CA166051/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- DBG-22-161-01-MM/American Cancer Society (American Cancer Society, Inc.)
LinkOut - more resources
Full Text Sources

