Aged bone marrow macrophages drive systemic aging and age-related dysfunction via extracellular vesicle-mediated induction of paracrine senescence
- PMID: 39266768
- PMCID: PMC11564114
- DOI: 10.1038/s43587-024-00694-0
Aged bone marrow macrophages drive systemic aging and age-related dysfunction via extracellular vesicle-mediated induction of paracrine senescence
Abstract
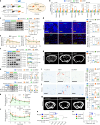
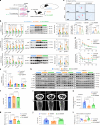
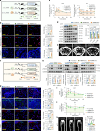
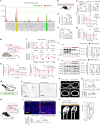
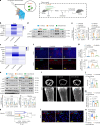
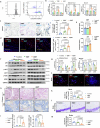
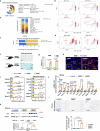
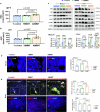
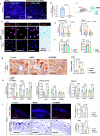
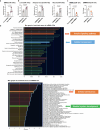
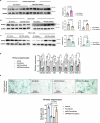
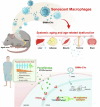
The accumulation and systemic propagation of senescent cells contributes to physiological aging and age-related pathology. However, which cell types are most susceptible to the aged milieu and could be responsible for the propagation of senescence has remained unclear. Here we found that physiologically aged bone marrow monocytes/macrophages (BMMs) propagate senescence to multiple tissues, through extracellular vesicles (EVs), and drive age-associated dysfunction in mice. We identified peroxisome proliferator-activated receptor α (PPARα) as a target of microRNAs within aged BMM-EVs that regulates downstream effects on senescence and age-related dysfunction. Demonstrating therapeutic potential, we report that treatment with the PPARα agonist fenofibrate effectively restores tissue homeostasis in aged mice. Suggesting conservation to humans, in a cohort study of 7,986 participants, we found that fenofibrate use is associated with a reduced risk of age-related chronic disease and higher life expectancy. Together, our findings establish that BMMs can propagate senescence to distant tissues and cause age-related dysfunction, and they provide supportive evidence for fenofibrate to extend healthy lifespan.
© 2024. The Author(s).
Conflict of interest statement
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

