Inhibition of CRMP2 Phosphorylation Suppresses Microglia Activation in the Retina and Optic Nerve and Promotes Optic Nerve Regeneration After Optic Nerve Injury
- PMID: 39266914
- PMCID: PMC11393028
- DOI: 10.1007/s12017-024-08805-1
Inhibition of CRMP2 Phosphorylation Suppresses Microglia Activation in the Retina and Optic Nerve and Promotes Optic Nerve Regeneration After Optic Nerve Injury
Abstract
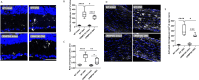
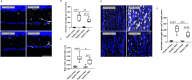
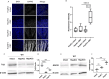
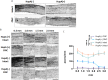
As the primary connection between the eye and brain, the optic nerve plays a pivotal role in visual information transmission. Injuries to the optic nerve can occur for various reasons, including trauma, glaucoma, and neurodegenerative diseases. Retinal ganglion cells (RGCs), a type of neurons that extend axons through the optic nerve, can rapidly respond to injury and initiate cell death. Additionally, following optic nerve injury microglia, which serve as markers of neuroinflammation, transition from a resting state to an activated state. The phosphorylation of collapsin response mediator protein2 (CRMP2) in the semaphorin 3A (Sema3A) signalling pathway affects several processes, including axon guidance and neuron regeneration. In this study, we used an optic nerve crush (ONC) mouse model to investigate the effects of suppressing CRMP2 phosphorylation on microglia activation. We found that CRMP2 phosphorylation inhibitor suppressed RGCs loss and promoted neuronal regeneration following ONC. In addition, CRMP2 S522A mutant (CRMP2 KI) mice exhibited decreased microglial activation in both the retina and optic nerve following ONC. These results suggest that inhibiting the phosphorylation of CRMP2 can alleviate the loss of RGCs and microglial activation after optic nerve injury, providing insight into the development of treatments for optical neuropathies and neurodegenerative diseases.
Keywords: Collapsin mediator protein 2; Microglia; Optic nerve injury; Phosphorylation.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that there is no conflict of interest regarding the publication of this paper.
Figures

Similar articles
-
Unraveling the Nexus: The Role of Collapsin Response Mediator Protein 2 Phosphorylation in Neurodegeneration and Neuroregeneration.Neuromolecular Med. 2024 Nov 12;26(1):45. doi: 10.1007/s12017-024-08814-0. Neuromolecular Med. 2024. PMID: 39532785 Free PMC article. Review.
-
Genetic inhibition of CRMP2 phosphorylation at serine 522 promotes axonal regeneration after optic nerve injury.Sci Rep. 2019 May 10;9(1):7188. doi: 10.1038/s41598-019-43658-w. Sci Rep. 2019. PMID: 31076621 Free PMC article.
-
Genetic inhibition of CRMP2 phosphorylation delays Wallerian degeneration after optic nerve injury.Biochem Biophys Res Commun. 2019 Jul 5;514(4):1037-1039. doi: 10.1016/j.bbrc.2019.05.060. Epub 2019 May 13. Biochem Biophys Res Commun. 2019. PMID: 31097218
-
Topical ripasudil stimulates neuroprotection and axon regeneration in adult mice following optic nerve injury.Sci Rep. 2020 Sep 24;10(1):15709. doi: 10.1038/s41598-020-72748-3. Sci Rep. 2020. PMID: 32973242 Free PMC article.
-
Neuroinflammation, Microglia and Implications for Retinal Ganglion Cell Survival and Axon Regeneration in Traumatic Optic Neuropathy.Front Immunol. 2022 Mar 4;13:860070. doi: 10.3389/fimmu.2022.860070. eCollection 2022. Front Immunol. 2022. PMID: 35309305 Free PMC article. Review.
Cited by
-
Protocol for the purification and culture of primary retinal ganglion cells and development of common pathological models.J Mol Histol. 2025 Aug 8;56(4):260. doi: 10.1007/s10735-025-10536-x. J Mol Histol. 2025. PMID: 40779178
-
Unraveling the Nexus: The Role of Collapsin Response Mediator Protein 2 Phosphorylation in Neurodegeneration and Neuroregeneration.Neuromolecular Med. 2024 Nov 12;26(1):45. doi: 10.1007/s12017-024-08814-0. Neuromolecular Med. 2024. PMID: 39532785 Free PMC article. Review.
-
Clinical and Scientific Considerations for Whole Eye Transplantation: An Ophthalmologist's Perspective.Transl Vis Sci Technol. 2025 Feb 3;14(2):13. doi: 10.1167/tvst.14.2.13. Transl Vis Sci Technol. 2025. PMID: 39918461 Free PMC article. No abstract available.
References
-
- Au, N. P. B., Kumar, G., Asthana, P., Gao, F., Kawaguchi, R., Chang, R. C. C., So, K. F., Hu, Y., Geschwind, D. H., Coppola, G., & Ma, C. H. E. (2022). Clinically relevant small-molecule promotes nerve repair and visual function recovery. Npj Regenerative Medicine,7(1), 50. 10.1038/s41536-022-00233-8 - PMC - PubMed
-
- Boczek, N. J., Ye, D., Johnson, E. K., Wang, W., Crotti, L., Tester, D. J., Dagradi, F., Mizusawa, Y., Torchio, M., Alders, M., Giudicessi, J. R., Wilde, A. A. M., Schwartz, P. J., Nerbonne, J. M., & Ackerman, M. J. (2014). Characterization of SEMA3A -encoded semaphorin as a naturally occurring K v 4.3 protein inhibitor and its contribution to brugada syndrome. Circulation Research,115(4), 460–469. 10.1161/CIRCRESAHA.115.303657 - PMC - PubMed
-
- Brahma, M. M., Takahashi, K., Namekata, K., Harada, T., Goshima, Y., & Ohshima, T. (2022). Genetic inhibition of collapsin response mediator protein-2 phosphorylation ameliorates retinal ganglion cell death in normal-tension glaucoma models. Genes to Cells,27(8), 526–536. 10.1111/gtc.12971 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

