Multi-omics analyses reveal the mechanisms underlying the responses of Casuarina equisetifolia ssp. incana to seawater atomization and encroachment stress
- PMID: 39266948
- PMCID: PMC11391710
- DOI: 10.1186/s12870-024-05561-z
Multi-omics analyses reveal the mechanisms underlying the responses of Casuarina equisetifolia ssp. incana to seawater atomization and encroachment stress
Abstract
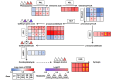
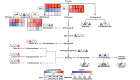
Casuarina equisetifolia trees are used as windbreaks in subtropical and tropical coastal zones, while C. equisetifolia windbreak forests can be degraded by seawater atomization (SA) and seawater encroachment (SE). To investigate the mechanisms underlying the response of C. equisetifolia to SA and SE stress, the transcriptome and metabolome of C. equisetifolia seedlings treated with control, SA, and SE treatments were analyzed. We identified 737, 3232, 3138, and 3899 differentially expressed genes (SA and SE for 2 and 24 h), and 46, 66, 62, and 65 differentially accumulated metabolites (SA and SE for 12 and 24 h). The Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis showed that SA and SE stress significantly altered the expression of genes related to plant hormone signal transduction, plant-pathogen interaction, and starch and sucrose metabolism pathways. The accumulation of metabolites associated with the biosynthetic pathways of phenylpropanoid and amino acids, as well as starch and sucrose metabolism, and glycolysis/gluconeogenesis were significantly altered in C. equisetifolia subjected to SA and SE stress. In conclusion, C. equisetifolia responds to SA and SE stress by regulating plant hormone signal transduction, plant-pathogen interaction, biosynthesis of phenylpropanoid and amino acids, starch and sucrose metabolism, and glycolysis/gluconeogenesis pathways. Compared with SA stress, C. equisetifolia had a stronger perception and response to SE stress, which required more genes and metabolites to be regulated. This study enhances our understandings of how C. equisetifolia responds to two types of seawater stresses at transcriptional and metabolic levels. It also offers a theoretical framework for effective coastal vegetation management in tropical and subtropical regions.
Keywords: Casuarina equisetifolia; Metabolome; Seawater atomization; Seawater encroachment; Transcriptome.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Transcriptome and metabolome analysis reveals key genes and secondary metabolites of Casuarina equisetifolia ssp. incana in response to drought stress.BMC Plant Biol. 2023 Apr 18;23(1):200. doi: 10.1186/s12870-023-04206-x. BMC Plant Biol. 2023. PMID: 37069496 Free PMC article.
-
Multi-omics analysis reveals insights into hypoxia-tolerant rice growth and identifies the 1-Cys peroxiredoxin B-like protease.Int J Biol Macromol. 2025 Jun;312:143953. doi: 10.1016/j.ijbiomac.2025.143953. Epub 2025 May 9. Int J Biol Macromol. 2025. PMID: 40350109
-
Endophytic bacteria with allelopathic potential regulate gene expression and metabolite production in host Casuarina equisetifolia.Front Plant Sci. 2024 Sep 18;15:1435440. doi: 10.3389/fpls.2024.1435440. eCollection 2024. Front Plant Sci. 2024. PMID: 39359630 Free PMC article.
-
Integrated Transcriptomic and Metabolomics Analyses Reveal Molecular Responses to Cold Stress in Coconut (Cocos nucifera L.) Seedlings.Int J Mol Sci. 2023 Sep 26;24(19):14563. doi: 10.3390/ijms241914563. Int J Mol Sci. 2023. PMID: 37834015 Free PMC article.
-
CeJAZ3 suppresses longifolene accumulation in Casuarina equisetifolia, affecting the host preference of Anoplophora chinensis.Pest Manag Sci. 2025 Apr;81(4):2202-2214. doi: 10.1002/ps.8618. Epub 2024 Dec 26. Pest Manag Sci. 2025. PMID: 39723485
References
-
- Cao B, Li N, Xu K. Crosstalk of phenylpropanoid biosynthesis with hormone signaling in Chinese cabbage is key to counteracting salt stress. Environ Exp Bot. 2020;179:104209.10.1016/j.envexpbot.2020.104209 - DOI
MeSH terms
Grants and funding
- 2021YFC3100401/National Key R&D Program of China
- 2021YFC3100401/National Key R&D Program of China
- 2021YFC3100401/National Key R&D Program of China
- 241801041/High-level Talent Research Start-up Project Funding of Henan Academy of Sciences
- 241801041/High-level Talent Research Start-up Project Funding of Henan Academy of Sciences
- 241801041/High-level Talent Research Start-up Project Funding of Henan Academy of Sciences
- 241801041/High-level Talent Research Start-up Project Funding of Henan Academy of Sciences
- 241801041/High-level Talent Research Start-up Project Funding of Henan Academy of Sciences
- 241801041/High-level Talent Research Start-up Project Funding of Henan Academy of Sciences
- 241801041/High-level Talent Research Start-up Project Funding of Henan Academy of Sciences
- 241801041/High-level Talent Research Start-up Project Funding of Henan Academy of Sciences
- 241801041/High-level Talent Research Start-up Project Funding of Henan Academy of Sciences
- 242102320323/Science and Technology Research Project of Henan Province
- 242102320323/Science and Technology Research Project of Henan Province
- 242102320323/Science and Technology Research Project of Henan Province
- 242102320323/Science and Technology Research Project of Henan Province
- 242102320323/Science and Technology Research Project of Henan Province
- 242102320323/Science and Technology Research Project of Henan Province
- 242102320323/Science and Technology Research Project of Henan Province
- 242102320323/Science and Technology Research Project of Henan Province
- 242102320323/Science and Technology Research Project of Henan Province
- 225200810104/Joint Fund of Henan Province Science and Technology R&D Program
- 225200810104/Joint Fund of Henan Province Science and Technology R&D Program
- 225200810104/Joint Fund of Henan Province Science and Technology R&D Program
- 225200810104/Joint Fund of Henan Province Science and Technology R&D Program
- 225200810104/Joint Fund of Henan Province Science and Technology R&D Program
- 225200810104/Joint Fund of Henan Province Science and Technology R&D Program
- 225200810104/Joint Fund of Henan Province Science and Technology R&D Program
- 225200810104/Joint Fund of Henan Province Science and Technology R&D Program
- 225200810104/Joint Fund of Henan Province Science and Technology R&D Program
- 2022KYXM09/Forestry Science and Technology Innovation Fund Project of Guangdong Province
- 2022KYXM09/Forestry Science and Technology Innovation Fund Project of Guangdong Province
- 2022KYXM09/Forestry Science and Technology Innovation Fund Project of Guangdong Province
- 2022KYXM09/Forestry Science and Technology Innovation Fund Project of Guangdong Province
- 2021A1515011670/Natural Science Foundation of Guangdong Province
- 2021A1515011670/Natural Science Foundation of Guangdong Province
- 2023B1212060046/Guangdong Science and Technology Plan Project
LinkOut - more resources
Full Text Sources

