Genetic variants associated with response to anti-CGRP monoclonal antibody therapy in a chronic migraine Han Chinese population
- PMID: 39266962
- PMCID: PMC11391721
- DOI: 10.1186/s10194-024-01850-y
Genetic variants associated with response to anti-CGRP monoclonal antibody therapy in a chronic migraine Han Chinese population
Abstract
Background: Anti-calcitonin gene-related peptide (CGRP) monoclonal antibodies have emerged as promising therapeutic options for the treatment of chronic migraine. However, treatment response varies considerably among individuals, suggesting a potential role for genetic factors. This study aimed to identify genetic variants affecting the efficacy of anti-CGRP monoclonal antibody therapy in chronic migraine among the Han Chinese population in Taiwan to enhance treatment precision and to understand the genetic architecture of migraine.
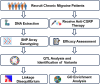
Methods: We conducted a quantitative trait locus (QTL) association study in patients with chronic migraines from a tertiary medical center in Taiwan using the Taiwan Precision Medicine Array Chip. The patients received fremanezumab or galcanezumab for at least 12 weeks. Treatment efficacy was assessed based on the improvement rate in monthly migraine days. Genetic variants were identified, and their associations with treatment efficacy were examined through quantitative trait loci analysis, linkage disequilibrium studies, and functional annotations using the Gene Ontology database.
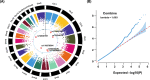
Results: Six single nucleotide polymorphisms (SNPs) relative variants were significantly associated with anti-CGRP therapy response (p < 1 × 10- 7): rs116870564, rs75244870, rs56216870, rs12938101, rs74655790, and rs149540851. These variants are located in or near genes, including LRRC4C, ATAD2B, and OXR1, which are involved in neuronal development, DNA-dependent ATPase activity, and oxidation-reduction processes, respectively. The rs116870564 variant in LRRC4C showed the strongest association (β = -0.551, p = 6.65 × 10- 9). The functional impact of these variants is attributed to their regulatory effects on gene expression, which are influenced by intron splicing regulation, transcription factors, and changes in chromatin structure.
Conclusion: The identification of key genetic markers associated with response to anti-CGRP therapy emphasizes the importance of genetic variability in treatment efficacy. This could lead to more personalized chronic migraine management strategies and tailored therapeutic approaches based on individual genetic profiles. Further research in larger, diverse populations is warranted to validate these findings and refine our understanding of the role of CGRP in chronic migraine pathophysiology.
Trial registration: Not applicable.
Keywords: Chronic migraine; Genetic variants; Personalized medicine; SNP genotyping; Treatment response; anti-CGRP monoclonal antibodies.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests. The study protocol was approved by the TSGH Institutional Review Board (TSGHIRB: 2-105-05-038). All individuals provided signed, informed consents before enrollment.
The authors declare no competing interests.
Figures


References
-
- Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia. (2018) ;38:1-211 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

