Longevity of hybrid immunity against SARS-CoV-2 in adults vaccinated with an adenovirus-based COVID-19 vaccine
- PMID: 39266969
- PMCID: PMC11391831
- DOI: 10.1186/s12879-024-09891-z
Longevity of hybrid immunity against SARS-CoV-2 in adults vaccinated with an adenovirus-based COVID-19 vaccine
Abstract
Background: Hybrid immunity provides better protection against COVID-19 than vaccination or prior natural infection alone. It induces high magnitude and broadly cross-reactive neutralising anti-Spike IgG antibodies. However, it is not clear how long these potent antibodies last, especially in the context of adenovirus-based COVID-19 vaccines.
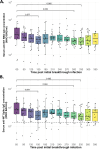
Methods: We conducted a longitudinal cohort study and enrolled 20 adults who had received an adenovirus-based COVID-19 vaccine before a laboratory-confirmed SARS-CoV-2 infection. We followed up the study participants for 390 days post the initial breakthrough infection. We assessed the longevity and cross-reactive breadth of serum antibodies against SARS-CoV-2 variants of concern (VOCs), including Omicron.
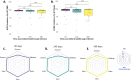
Results: The binding anti-Spike IgG antibodies remained within the reported putative levels for at least 360 days and were cross-neutralising against Beta, Gamma, Delta, and Omicron. During the follow up period, a median of one SARS-CoV-2 re-infection event was observed across the cohort, but none resulted in severe COVID-19. Moreover, the re-exposure events were associated with augmented anti-Spike and anti-RBD IgG antibody titres.
Conclusions: This study confirms that hybrid immunity provides durable broadly cross-reactive antibody immunity against SARS-CoV-2 variants of concern for at least a year (360 days), and that it is further augment by SARS-CoV-2 re-exposure.
Keywords: Breakthrough infection; Hybrid immunity; Longevity; SARS-CoV-2.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- COVID-19 Case Tracker [https://coronavirus.jhu.edu/map.html.].
-
- Cromer D, Steain M, Reynaldi A, Schlub TE, Wheatley AK, Juno JA, Kent SJ, Triccas JA, Khoury DS, Davenport MP. Neutralising antibody titres as predictors of protection against SARS-CoV-2 variants and the impact of boosting: a meta-analysis. Lancet Microbe. 2022;3(1):e52–61. 10.1016/S2666-5247(21)00267-6 - DOI - PMC - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

