Development of the Better Research Interactions for Every Family (BRIEF) intervention to support recruitment for neonatal clinical trials: an intervention mapping guided approach
- PMID: 39267164
- PMCID: PMC11395641
- DOI: 10.1186/s13063-024-08446-6
Development of the Better Research Interactions for Every Family (BRIEF) intervention to support recruitment for neonatal clinical trials: an intervention mapping guided approach
Abstract
Background: Recruitment for neonatal clinical trials can be particularly challenging. Low enrollment rates bias the research population and decrease generalizability of findings. We identified a critical need for an intervention to improve how researchers recruit for neonatal clinical trials. Working within the US neonatal research context, we developed the Better Research Interactions for Every Family (BRIEF) Intervention, which had two overarching goals: to improve the recruitment experience for all parents, focusing on minoritized populations, and to increase participation, focusing on decreasing disparities in research participation.
Methods: We used intervention mapping (IM) to guide all steps of intervention development. IM is a planning framework that provides a systematic process and detailed protocol for step-by-step decision-making for intervention development, implementation, and evaluation.
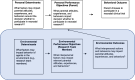
Results: We performed IM's six steps. In step 1, we convened two stakeholder groups, a parent panel and an expert panel, who provided guidance through development of all BRIEF components. Through a recent systematic review, empirical data collected by our team, and consultations with the panels, we identified key determinants (barriers and facilitators) of low enrollment rates and research team members as change agents. In step 2, we iteratively refined our list of key factors to include and linked determinants of behavior changes to these performance objectives. In step 3, we chose three theories (social cognitive theory, theory of information processing, and the trans-theoretical model), methods from identified practical applications suitable for the population (research team members) and the context (busy research NICU teams). In step 4, we developed and refined the intervention components, including self-guided pre-work and a single in-person session. In step 5, we identified the Darbepoetin plus slow-release intravenous iron trial as our partner study in which to pilot BRIEF. In step 6, we developed a multi-stage evaluation plan that included five distinct levels of outcomes.
Conclusions: This manuscript shares our rationale and processes for the creation of a research team member-facing intervention aiming to improve recruitment processes for neonatal clinical trials. Our approach can inform those aiming to improve recruitment for neonatal clinical trials and those who may be considering use of IM within similar contexts.
Keywords: Intervention; Neonatal clinical trials; Recruitment; Research ethics.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Recruitment of racial and ethnic minorities to clinical trials conducted within specialty clinics: an intervention mapping approach.Trials. 2018 Feb 17;19(1):115. doi: 10.1186/s13063-018-2507-9. Trials. 2018. PMID: 29454389 Free PMC article.
-
Time to STEP UP: methods and findings from the development of guidance to help researchers design inclusive clinical trials.BMC Med Res Methodol. 2024 Oct 2;24(1):227. doi: 10.1186/s12874-024-02342-y. BMC Med Res Methodol. 2024. PMID: 39358688 Free PMC article.
-
The future of Cochrane Neonatal.Early Hum Dev. 2020 Nov;150:105191. doi: 10.1016/j.earlhumdev.2020.105191. Epub 2020 Sep 12. Early Hum Dev. 2020. PMID: 33036834
-
Impact of summer programmes on the outcomes of disadvantaged or 'at risk' young people: A systematic review.Campbell Syst Rev. 2024 Jun 13;20(2):e1406. doi: 10.1002/cl2.1406. eCollection 2024 Jun. Campbell Syst Rev. 2024. PMID: 38873396 Free PMC article. Review.
-
Cue-based versus scheduled feeding for preterm infants transitioning from tube to oral feeding: the Cubs mixed-methods feasibility study.Health Technol Assess. 2021 Dec;25(74):1-146. doi: 10.3310/hta25740. Health Technol Assess. 2021. PMID: 34878383
Cited by
-
Eligible Infants Included in Neonatal Clinical Trials and Reasons for Noninclusion: A Systematic Review.JAMA Netw Open. 2024 Oct 1;7(10):e2441372. doi: 10.1001/jamanetworkopen.2024.41372. JAMA Netw Open. 2024. PMID: 39453652 Free PMC article.
References
-
- Williams RJ, Tse T, DiPiazza K, Zarin DA. Terminated trials in the ClinicalTrials.gov Results database: evaluation of availability of primary outcome data and reasons for termination. PLoS One. 2015;10(5):e0127242. 10.1371/journal.pone.0127242. 10.1371/journal.pone.0127242 - DOI - PMC - PubMed
-
- Walters SJ, dos Anjos Henriques-Cadby IB, Bortolami O, et al. Recruitment and retention of participants in randomised controlled trials: a review of trials funded and published by the United Kingdom Health Technology Assessment Programme. BMJ Open. 2017;7(3):e015276. 10.1136/bmjopen-2016-015276. 10.1136/bmjopen-2016-015276 - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

