Impact of amyloid and cardiometabolic risk factors on prognostic capacity of plasma neurofilament light chain for neurodegeneration
- PMID: 39267169
- PMCID: PMC11397040
- DOI: 10.1186/s13195-024-01564-y
Impact of amyloid and cardiometabolic risk factors on prognostic capacity of plasma neurofilament light chain for neurodegeneration
Abstract
Background: Plasma neurofilament light chain (NfL) is a blood biomarker of neurodegeneration, including Alzheimer's disease. However, its usefulness may be influenced by common conditions in older adults, including amyloid-β (Aβ) deposition and cardiometabolic risk factors like hypertension, diabetes mellitus (DM), impaired kidney function, and obesity. This longitudinal observational study using the Alzheimer's Disease Neuroimaging Initiative cohort investigated how these conditions influence the prognostic capacity of plasma NfL.
Methods: Non-demented participants (cognitively unimpaired or mild cognitive impairment) underwent repeated assessments including the Alzheimer's Disease Assessment Scale-Cognitive subscale (ADAS-Cog) scores, hippocampal volumes, and white matter hyperintensity (WMH) volumes at 6- or 12-month intervals. Linear mixed-effect models were employed to examine the interaction between plasma NfL and various variables of interest, such as Aβ (evaluated using Florbetapir positron emission tomography), hypertension, DM, impaired kidney function, or obesity.
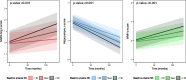
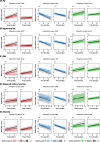
Results: Over a mean follow-up period of 62.5 months, participants with a mean age of 72.1 years (n = 720, 48.8% female) at baseline were observed. Higher plasma NfL levels at baseline were associated with steeper increases in ADAS-Cog scores and WMH volumes, and steeper decreases in hippocampal volumes over time (all p-values < 0.001). Notably, Aβ at baseline significantly enhanced the association between plasma NfL and longitudinal changes in ADAS-Cog scores (p-value 0.005) and hippocampal volumes (p-value 0.004). Regarding ADAS-Cog score and WMH volume, the impact of Aβ was more prominent in cognitively unimpaired than in mild cognitive impairment. Hypertension significantly heightened the association between plasma NfL and longitudinal changes in ADAS-Cog scores, hippocampal volumes, and WMH volumes (all p-values < 0.001). DM influenced the association between plasma NfL and changes in ADAS-Cog scores (p-value < 0.001) without affecting hippocampal and WMH volumes. Impaired kidney function did not significantly alter the association between plasma NfL and longitudinal changes in any outcome variables. Obesity heightened the association between plasma NfL and changes in hippocampal volumes only (p-value 0.026).
Conclusion: This study suggests that the prognostic capacity of plasma NfL may be amplified in individuals with Aβ or hypertension. This finding emphasizes the importance of considering these factors in the NfL-based prognostic model for neurodegeneration in non-demented older adults.
Keywords: Alzheimer’s disease; Blood-based biomarker; Cardiovascular disease; Dementia; Kidney disease; Metabolic syndrome; Neurofilament light chain; Prognosis.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Mazzeo S, Ingannato A, Giacomucci G, Manganelli A, Moschini V, Balestrini J, et al. Plasma neurofilament light chain predicts Alzheimer’s disease in patients with subjective cognitive decline and mild cognitive impairment: a cross-sectional and longitudinal study. Eur J Neurol. 2023. 10.1111/ene.16089. 10.1111/ene.16089 - DOI - PMC - PubMed

