Antibody-Mediated Rejection in Liver Transplantation: Immuno-Pathological Characteristics and Long-Term Follow-Up
- PMID: 39267618
- PMCID: PMC11391112
- DOI: 10.3389/ti.2024.13232
Antibody-Mediated Rejection in Liver Transplantation: Immuno-Pathological Characteristics and Long-Term Follow-Up
Abstract
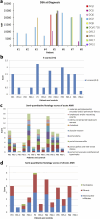
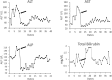
The diagnosis of liver antibody-mediated rejection (AMR) is challenging and likely under-recognized. The association of AMR with donor-specific antibodies (DSA), and its clinical course in relation to pathologic findings and treatment are ill defined. We identified cases of liver AMR by following the criteria outlined by the 2016 Banff Working Group. Patient demographics, native liver disease, histopathologic findings, treatment type, clinical outcome, and transaminase levels during AMR diagnosis, treatment, and resolution were determined. Patients (n = 8) with AMR average age was 55.2 years (range: 19-68). Seven of eight cases met the Banff criteria for AMR. Personalized treatment regimens consisted of optimization of immunosuppression, intravenous pulse steroids, plasmapheresis, IVIG, rituximab, and bortezomib. Five patients experienced complete resolution of AMR, return of transaminases to baseline, and decreased DSA at long-term follow-up. One patient developed chronic AMR and two patients required re-transplantation. Follow-up after AMR diagnosis ranged from one to 11 years. Because AMR can present at any time, crossmatch, early biopsy, and routine monitoring of DSA levels should be implemented following transaminase elevation to recognize AMR. Furthermore, treatment should be immediately implemented to reverse AMR and prevent graft failure, chronic damage, re-transplantation, and possibly mortality.
Keywords: AMR; C4d; DSA; allograft; rejection; solid organ transplant.
Copyright © 2024 Cicalese, Walton, Du, Kulkarni, Qiu, El Hag and Stevenson.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

