Theoretical basis, state and challenges of living cell-based drug delivery systems
- PMID: 39267776
- PMCID: PMC11388066
- DOI: 10.7150/thno.99257
Theoretical basis, state and challenges of living cell-based drug delivery systems
Abstract
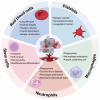
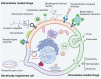
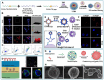
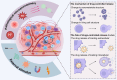
The therapeutic efficacy of drugs is determined, to a certain extent, by the efficiency of drug delivery. The low efficiency of drug delivery systems (DDSs) is frequently associated with serious toxic side effects and can even prove fatal in certain cases. With the rapid development of technology, drug delivery has evolved from using traditional frameworks to using nano DDSs (NDDSs), endogenous biomaterials DDSs (EBDDSs), and living cell DDSs (LCDDSs). LCDDSs are receiving widespread attention from researchers at present owing to the unique advantages of living cells in targeted drug delivery, including their excellent biocompatibility properties, low immunogenicity, unique biological properties and functions, and role in the treatment of diseases. However, the theoretical basis and techniques involved in the application of LCDDSs have not been extensively summarized to date. Therefore, this review comprehensively summarizes the properties and applications of living cells, elaborates the various drug loading approaches and controlled drug release, and discusses the results of clinical trials. The review also discusses the current shortcomings and prospects for the future development of LCDDSs, which will serve as highly valuable insights for the development and clinical transformation of LCDDSs in the future.
Keywords: Clinical transformation; Controlled drug release; Drug loading approaches; Living cells; Targeted drug delivery.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

