Mitochondria-encoded peptide MOTS-c participates in plasma membrane repair by facilitating the translocation of TRIM72 to membrane
- PMID: 39267782
- PMCID: PMC11388074
- DOI: 10.7150/thno.100321
Mitochondria-encoded peptide MOTS-c participates in plasma membrane repair by facilitating the translocation of TRIM72 to membrane
Abstract
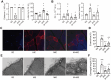
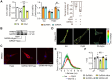
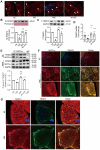
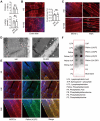
Rationale: An impairment of plasma membrane repair has been implicated in various diseases such as muscular dystrophy and ischemia/reperfusion injury. MOTS-c, a short peptide encoded by mitochondria, has been shown to pass through the plasma membrane into the bloodstream. This study determined whether this biological behavior was involved in membrane repair and its underlying mechanism. Methods and Results: In human participants, the level of MOTS-c was positively correlated with the abundance of mitochondria, and the membrane repair molecule TRIM72. In contrast to high-intensity eccentric exercise, moderate-intensity exercise improved sarcolemma integrity and physical performance, accompanied by an increase of mitochondria beneath the damaged sarcolemma and secretion of MOTS-c. Furthermore, moderate-intensity exercise increased the interaction between MOTS-c and TRIM72, and MOTS-c facilitated the trafficking of TRIM72 to the sarcolemma. In vitro studies demonstrated that MOTS-c attenuated membrane damage induced by hypotonic solution, which could be blocked by siRNA-TRIM72, but not AMPK inhibitor. Co-immunoprecipitation study showed that MOTS-c interacted with TRIM72 C-terminus, but not N-terminus. The dynamic membrane repair assay revealed that MOTS-c boosted the trafficking of TRIM72 to the injured membrane. However, MOTS-c itself had negligible effects on membrane repair, which was recapitulated in TRIM72-/- mice. Unexpectedly, MOTS-c still increased the fusion of vesicles with the membrane in TRIM72-/- mice, and dot blot analysis revealed an interaction between MOTS-c and phosphatidylinositol (4,5) bisphosphate [PtdIns (4,5) P2]. Finally, MOTS-c blunted ischemia/reperfusion-induced membrane disruption, and preserved heart function. Conclusions: MOTS-c/TRIM72-mediated membrane integrity improvement participates in mitochondria-triggered membrane repair. An interaction between MOTS-c and plasma lipid contributes to the fusion of vesicles with membrane. Our data provide a novel therapeutic strategy for rescuing organ function by facilitating membrane repair with MOTS-c.
Keywords: Ischemia/reperfusion injury; MOTS-c; Membrane repair; Mitochondria; TRIM72.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

