Immunomodulatory effects of live and UV-killed Bacillus subtilis natto on inflammatory response in human colorectal adenocarcinoma cell line in vitro
- PMID: 39267934
- PMCID: PMC11389770
- DOI: 10.18502/ijm.v16i4.16301
Immunomodulatory effects of live and UV-killed Bacillus subtilis natto on inflammatory response in human colorectal adenocarcinoma cell line in vitro
Abstract
Background and objectives: Colorectal cancer (CRC) is a heterogeneous disease of the colon or rectum arising from adenoma precursors and serrated polyps. Recently, probiotics have been proposed as an effective and potential therapeutic approach for CRC prevention and treatment. Probiotics have been shown to alleviate inflammation by restoring the integrity of the mucosal barrier and impeding cancer progression.
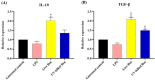
Materials and methods: In this study, we aimed to investigate the immunomodulatory effects of live and UV-killed Bacillus subtilis natto on the inflammatory response in CRC. Caco-2 cells were exposed to various concentrations of live and UV- killed B. subtilis natto, and cell viability was assessed using MTT assay. Gene expression analysis of IL-10, TGF-β, TLR2 and TLR4 was performed using RT-qPCR.
Results: Our findings showed that both live and UV-killed B. subtilis natto caused significant reduction in inflammatory response by decreasing the gene expression of TLR2 and TLR4, and enhancing the gene expression of IL-10 and TGF-β in Caco-2 cells as compared to control group.
Conclusion: The results of this study suggest that live and UV-killed B. subtilis natto may hold potential as a therapeutic supplement for modulating inflammation in CRC.
Keywords: Bacillus subtilis; Colorectal neoplasms; Gene expression; Immunomodulation; Probiotics.
Copyright© 2024 The Authors. Published by Tehran University of Medical Sciences.
Figures


References
-
- Ciardiello F, Ciardiello D, Martini G, Napolitano S, Tabernero J, Cervantes A. Clinical management of metastatic colorectal cancer in the era of precision medicine. CA Cancer J Clin 2022; 72: 372–401. - PubMed
-
- Onyoh EF, Hsu W-F, Chang L-C, Lee Y-C, Wu M-S, Chiu H-M. The rise of colorectal cancer in Asia: epidemiology, screening, and management. Curr Gastroenterol Rep 2019; 21: 36. - PubMed
-
- Schmitt M, Greten FR. The inflammatory pathogenesis of colorectal cancer. Nat Rev Immunol 2021; 21: 653–667. - PubMed
LinkOut - more resources
Full Text Sources
