Target engagement of the subgenual anterior cingulate cortex with transcranial temporal interference stimulation in major depressive disorder: a protocol for a randomized sham-controlled trial
- PMID: 39268031
- PMCID: PMC11390435
- DOI: 10.3389/fnins.2024.1390250
Target engagement of the subgenual anterior cingulate cortex with transcranial temporal interference stimulation in major depressive disorder: a protocol for a randomized sham-controlled trial
Abstract
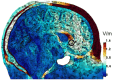
Background: Transcranial temporal interference stimulation (tTIS) is a new, emerging neurostimulation technology that utilizes two or more electric fields at specific frequencies to modulate the oscillations of neurons at a desired spatial location in the brain. The physics of tTIS offers the advantage of modulating deep brain structures in a non-invasive fashion and with minimal stimulation of the overlying cortex outside of a selected target. As such, tTIS can be effectively employed in the context of therapeutics for the psychiatric disease of disrupted brain connectivity, such as major depressive disorder (MDD). The subgenual anterior cingulate cortex (sgACC), a key brain center that regulates human emotions and influences negative emotional states, is a plausible target for tTIS in MDD based on reports of its successful neuromodulation with invasive deep brain stimulation.
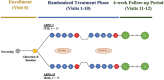
Methods: This pilot, single-site, double-blind, randomized, sham-controlled interventional clinical trial will be conducted at St. Michael's Hospital - Unity Health Toronto in Toronto, ON, Canada. The primary objective is to demonstrate target engagement of the sgACC with 130 Hz tTIS using resting-state magnetic resonance imaging (MRI) techniques. The secondary objective is to estimate the therapeutic potential of tTIS for MDD by evaluating the change in clinical characteristics of participants and electrophysiological outcomes and providing feasibility and tolerability estimates for a large-scale efficacy trial. Thirty participants (18-65 years) with unipolar, non-psychotic MDD will be recruited and randomized to receive 10 sessions of 130 Hz tTIS or sham stimulation (n = 15 per arm). The trial includes a pre- vs. post-treatment 3T MRI scan of the brain, clinical evaluation, and electroencephalography (EEG) acquisition at rest and during the auditory mismatch negativity (MMN) paradigm.
Discussion: This study is one of the first-ever clinical trials among patients with psychiatric disorders examining the therapeutic potential of repetitive tTIS and its neurobiological mechanisms. Data obtained from this trial will be used to optimize the tTIS approach and design a large-scale efficacy trial. Research in this area has the potential to provide a novel treatment option for individuals with MDD and circuitry-related disorders and may contribute to the process of obtaining regulatory approval for therapeutic applications of tTIS.
Clinical trial registration: ClinicalTrials.gov, identifier NCT05295888.
Keywords: brain stimulation; clinical trials; electric stimulation therapy; electroencephalography; feasibility studies; magnetic resonance imaging; mood disorders; temporal interference.
Copyright © 2024 Demchenko, Rampersad, Datta, Horn, Churchill, Kennedy, Krishnan, Rueda, Schweizer, Griffiths, Boyden, Santarnecchi and Bhat.
Conflict of interest statement
AD is an employee of Soterix Medical, Inc. AH was supported by the German Research Foundation (Deutsche Forschungsgemeinschaft, 424778381 – TRR 295), Deutsches Zentrum für Luft- und Raumfahrt (DynaSti grant within the EU Joint Programme Neurodegenerative Disease Research, JPND), the National Institutes of Health (R01 13478451, 1R01NS127892-01, 2R01 MH113929, and UM1NS132358) as well as the New Venture Fund (FFOR Seed Grant), and reports lecture fees for Boston Scientific and is a consultant for Functional Neuromodulation Ltd. and Abbott. SHK has received honoraria or research funds from Abbott, Alkermes, Allergan, Boehringer Ingelheim, Brain Canada, Canadian Institutes of Health Research, Janssen, Lundbeck, Lundbeck Institute, Ontario Brain Institute, Ontario Research Fund, Otsuka, Pfizer, Servier, Sunovion, Sun Pharmaceuticals, and holds stock in Field Trip Health. ESB is an inventor on the Temporal Interference (TI) Stimulation patent and a co-founder of TI Solutions, a for-profit company. VB is supported by the Academic Scholar Award from the University of Toronto Department of Psychiatry and has received research support from the Canadian Institutes of Health Research, Brain & Behavior Foundation, Ontario Ministry of Health Innovation Funds, Royal College of Physicians and Surgeons of Canada, Department of National Defence (Government of Canada), New Frontiers in Research Fund, Associated Medical Services Inc. Healthcare, American Foundation for Suicide Prevention, Roche Canada, Novartis, and Eisai. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Al-Arfaj H. K., Al-Sharydah A. M., AlSuhaibani S. S., Alaqeel S., Yousry T. (2023). Task-based and resting-state functional mri in observing eloquent cerebral areas personalized for epilepsy and surgical oncology patients: A review of the current evidence. JPM 13:370. 10.3390/jpm13020370 - DOI - PMC - PubMed
-
- Alexander M. L., Alagapan S., Lugo C. E., Mellin J. M., Lustenberger C., Rubinow D. R., et al. (2019). Double-blind, randomized pilot clinical trial targeting alpha oscillations with transcranial alternating current stimulation (tACS) for the treatment of major depressive disorder (MDD). Transl. Psychiatry 9:106. 10.1038/s41398-019-0439-0 - DOI - PMC - PubMed
-
- Anderson I., Ferrier I., Baldwin R., Cowen P., Howard L., Lewis G., et al. (2008). Evidence-based guidelines for treating depressive disorders with antidepressants: A revision of the 2000 British association for psychopharmacology guidelines. J. Psychopharmacol. 22 343–396. 10.1177/0269881107088441 - DOI - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

