Enteric glial cell diversification is influenced by spatiotemporal factors and source of neural progenitors in mice
- PMID: 39268038
- PMCID: PMC11390640
- DOI: 10.3389/fnins.2024.1392703
Enteric glial cell diversification is influenced by spatiotemporal factors and source of neural progenitors in mice
Abstract
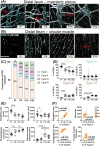
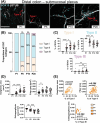
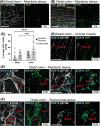
Previously focused primarily on enteric neurons, studies of the enteric nervous system (ENS) in both health and disease are now broadening to recognize the equally significant role played by enteric glial cells (EGCs). Commensurate to the vast array of gastrointestinal functions they influence, EGCs exhibit considerable diversity in terms of location, morphology, molecular profiles, and functional attributes. However, the mechanisms underlying this diversification of EGCs remain largely unexplored. To begin unraveling the mechanistic complexities of EGC diversity, the current study aimed to examine its spatiotemporal aspects in greater detail, and to assess whether the various sources of enteric neural progenitors contribute differentially to this diversity. Based on established topo-morphological criteria for categorizing EGCs into four main subtypes, our detailed immunofluorescence analyses first revealed that these subtypes emerge sequentially during early postnatal development, in a coordinated manner with the structural changes that occur in the ENS. When combined with genetic cell lineage tracing experiments, our analyses then uncovered a strongly biased contribution by Schwann cell-derived enteric neural progenitors to particular topo-morphological subtypes of EGCs. Taken together, these findings provide a robust foundation for further investigations into the molecular and cellular mechanisms governing EGC diversity.
Keywords: cell lineage tracing; enteric glial cells; enteric nervous system; myenteric plexus; postnatal development; schwann cell precursors; submucosal plexus; topo-morphological subtypes.
Copyright © 2024 Lefèvre, Godefroid, Soret and Pilon.
Conflict of interest statement
RS and NP are co-founders of the biotech company Neurenati Therapeutics, which had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures



References
-
- Baghdadi M., Kim T. (2023). The multiple roles of enteric glial cells in intestinal homeostasis and regeneration. Semin. Cell Dev. Biol. 150-151 43–49. - PubMed
-
- Boesmans W., Lasrado R., Vanden Berghe P., Pachnis V. (2015). Heterogeneity and phenotypic plasticity of glial cells in the mammalian enteric nervous system. Glia 63 229–241. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

