Comparison of four aortic bioprostheses: Hancock II vs. St Jude Trifecta vs. Carpentier-Edwards Perimount Magna vs. Magna Ease-mid-term results (COMPARE SAVR study)
- PMID: 39268099
- PMCID: PMC11388261
- DOI: 10.21037/jtd-22-1761
Comparison of four aortic bioprostheses: Hancock II vs. St Jude Trifecta vs. Carpentier-Edwards Perimount Magna vs. Magna Ease-mid-term results (COMPARE SAVR study)
Abstract
Background: In the era of percutaneous aortic valve implantation, biological valves are the preferred prostheses implanted in patients undergoing surgical aortic valve replacement (sAVR). The aim was to present a real-life analysis of mid-term sAVR outcomes for the four aortic bioprostheses: the Hancock II, the Carpentier-Edwards Perimount Magna, the Carpentier-Edwards Perimount Magna Ease and the Trifecta valve.
Methods: This is a retrospective study based on data from the Polish National Cardiac Surgery Database. The study population comprised of 1,589 consecutive patients, of whom 432 were in the Hancock II group, 356 in the Carpentier-Edwards Perimount Magna group, 427 in the Carpentier-Edwards Magna Ease group, and 374 in the Trifecta group. A comparison of the four groups was performed using analysis of variance (ANOVA) or Kruskal-Wallis test with appropriate post hoc tests (Tukey HSD or Steel-Dwass, respectively).
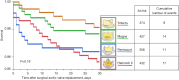
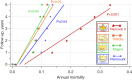
Results: Patients in the Hancock II group were older, had higher New York Heart Association (NYHA) and Canadian Cardiovascular Society (CCS) classes, had lower prevalence of hypertension and hyperlipidemia but higher prevalence of diabetes. The lowest mean valve size was observed in Trifecta group and the highest was in the Magna group (P<0.001). Survival analysis showed no significant differences in in-hospital mortality: 3.9% in Hancock II, 3.1% in Perimount, 3.3% in Magna and 2.1% in Trifecta group. Five-year mortality was significantly higher in Hancock II group (25.7%) compared to the other bioprostheses: 12.1% in Perimount, 9.1% in Magna and 10.70% in Trifecta group respectively.
Conclusions: The 5-year mortality rate was significantly higher in the Hancock II group compared to the other bioprostheses. In contrast, Trifecta, Perimount Magna, and Magna Ease had similar 5-year mortality rates.
Keywords: Bioprosthesis; Hancock II; Magna; Trifecta.
2024 Journal of Thoracic Disease. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1761/coif). The authors have no conflicts of interest to declare.
Figures


References
-
- Otto CM, Nishimura RA, Bonow RO, et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021;143:e35-71. 10.1161/CIR.0000000000000932 - DOI - PubMed
LinkOut - more resources
Full Text Sources
