Effect on post-operative pulmonary complications frequency of high flow nasal oxygen versus standard oxygen therapy in patients undergoing esophagectomy for cancer: study protocol for a randomized controlled trial-OSSIGENA study
- PMID: 39268119
- PMCID: PMC11388233
- DOI: 10.21037/jtd-24-575
Effect on post-operative pulmonary complications frequency of high flow nasal oxygen versus standard oxygen therapy in patients undergoing esophagectomy for cancer: study protocol for a randomized controlled trial-OSSIGENA study
Abstract
Background: Postoperative pulmonary complications (PPCs) remain a challenge after esophagectomy. Despite improvement in surgical and anesthesiological management, PPCs are reported in as many as 40% of patients. The main aim of this study is to investigate whether early application of high-flow nasal cannula (HFNC) after extubation will provide benefit in terms of reduced PPC frequency compared to standard oxygen therapy.
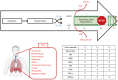
Methods: Patients aged 18-85 years undergoing esophagectomy for cancer treatment with radical intent, excluding those with American Society of Anesthesiologists (ASA) score >3 and severe systemic comorbidity (cardiac, pulmonary, renal or hepatic disease) will be randomized at the end of surgery to receive HFNC or standard oxygen therapy (Venturi mask or nasal goggles) after early extubation (within 12 hours after the end of surgery) for 48 hours. The main postoperative goals are to obtain SpO2 ≥94% and adequate pain control. Oxygen therapy after 48 hours will be stopped unless the physician deems it necessary. In case of respiratory clinical worsening, patients will be supported with the most appropriate tool (noninvasive ventilation or invasive mechanical ventilation). Pulmonary [pneumonia, pleural effusion, pneumothorax, atelectasis, acute respiratory distress syndrome (ARDS), tracheo-bronchial injury, air leak, reintubation, and/or respiratory failure] complications will be recorded as main outcome. Secondary outcomes, including cardiovascular, surgical, renal and infective complications will also be recorded. The primary analysis will be carried out on 320 patients (160 per group) and performed on an intention-to-treat (ITT) basis, including all participants randomized into the treatment groups, regardless of protocol adherence. The primary outcome, the PPC rate, will be compared between the two treatment groups using a chi-square test for categorical data, or Fisher's exact test will be used if the assumptions for the chi-square test are not met.
Discussion: Recent evidence demonstrated that early application of HFNC improved the respiratory rate oxygenation index (ROX index) after esophagectomy but did not reduce PPCs. This randomized controlled multicenter trial aims to assess the potential effect of the application of HFNC versus standard oxygen over PPCs in patients undergoing esophagectomy.
Trial registration: This study is registered at clinicaltrial.gov NCT05718284, dated 30 January 2023.
Keywords: Esophagectomy; high-flow nasal cannula (HFNC); outcome; perioperative medicine; postoperative pulmonary complications (PPCs).
2024 Journal of Thoracic Disease. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-575/coif). The authors have no conflicts of interest to declare.
Figures
References
-
- Morgan E, Soerjomataram I, Rumgay H, et al. The Global Landscape of Esophageal Squamous Cell Carcinoma and Esophageal Adenocarcinoma Incidence and Mortality in 2020 and Projections to 2040: New Estimates From GLOBOCAN 2020. Gastroenterology 2022;163:649-658.e2. 10.1053/j.gastro.2022.05.054 - DOI - PubMed
-
- Kuppusamy MK, Low DE, International Esodata Study Group (IESG) . Evaluation of International Contemporary Operative Outcomes and Management Trends Associated With Esophagectomy: A 4-Year Study of >6000 Patients Using ECCG Definitions and the Online Esodata Database. Ann Surg 2022;275:515-25. 10.1097/SLA.0000000000004309 - DOI - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
