Computational Study of the Ir-Catalyzed Formation of Allyl Carbamates from CO2
- PMID: 39268181
- PMCID: PMC11388460
- DOI: 10.1021/acs.organomet.4c00177
Computational Study of the Ir-Catalyzed Formation of Allyl Carbamates from CO2
Abstract
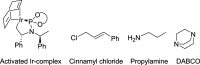
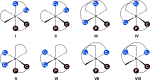
We have employed computational methods to investigate the iridium-catalyzed allylic substitution leading to the formation of enantioenriched allyl carbamates from carbon dioxide (CO2). The reaction occurs in several steps, with initial formation of an iridium-allyl, followed by nucleophilic attack by the carbamate formed in situ from CO2 and an amine. A detailed isomeric analysis shows that the rate-determining step differs for the (R)- and (S)-pathways. These insights are essential for understanding reactions involving enantioselective formation of allyl carbamates from CO2.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





Similar articles
-
Origins of enantioselectivity during allylic substitution reactions catalyzed by metallacyclic iridium complexes.J Am Chem Soc. 2012 May 16;134(19):8136-47. doi: 10.1021/ja212217j. Epub 2012 May 7. J Am Chem Soc. 2012. PMID: 22486270 Free PMC article.
-
Enantioselective transformation of allyl carbonates into branched allyl carbamates by using amines and recycling CO2 under iridium catalysis.Chemistry. 2014 Jun 10;20(24):7216-21. doi: 10.1002/chem.201402388. Epub 2014 May 18. Chemistry. 2014. PMID: 24839012
-
Chiral Amines via Enantioselective π-Allyliridium-C,O-Benzoate-Catalyzed Allylic Alkylation: Student Training via Industrial-Academic Collaboration.Acc Chem Res. 2022 Aug 2;55(15):2138-2147. doi: 10.1021/acs.accounts.2c00302. Epub 2022 Jul 13. Acc Chem Res. 2022. PMID: 35830564 Free PMC article.
-
CO2 carbamylation of proteins as a mechanism in physiology.Biochem Soc Trans. 2015 Jun;43(3):460-4. doi: 10.1042/BST20150026. Biochem Soc Trans. 2015. PMID: 26009191 Review.
-
Applications of Transition-Metal-Catalyzed Asymmetric Allylic Substitution in Total Synthesis of Natural Products: An Update.Chem Rec. 2021 Jan;21(1):29-68. doi: 10.1002/tcr.202000086. Epub 2020 Nov 18. Chem Rec. 2021. PMID: 33206466 Review.
References
-
- Le Quéré C.; Jackson R. B.; Jones M. W.; Smith A. J. P.; Abernethy S.; Andrew R. M.; De-Gol A. J.; Willis D. R.; Shan Y.; Canadell J. G.; Friedlingstein P.; Creutzig F.; Peters G. P. Temporary Reduction in Daily Global CO2 Emissions during the COVID-19 Forced Confinement. Nat. Clim. Change 2020, 10 (7), 647–653. 10.1038/s41558-020-0797-x. - DOI
-
- IEA . World Energy Outlook 2021; IEA: Paris; 2021. https://www.iea.org/reports/world-energy-outlook-2021.
-
- Blaser H.-U.; Federsel H.-J.. Asymmetric Catalysis on Industrial Scale: Challenges, Approaches and Solutions; Wiley-VCH, 2010.
LinkOut - more resources
Full Text Sources
