Integrated MXene and metal oxide electrocatalysts for the oxygen evolution reaction: synthesis, mechanisms, and advances
- PMID: 39268209
- PMCID: PMC11388099
- DOI: 10.1039/d4sc04141k
Integrated MXene and metal oxide electrocatalysts for the oxygen evolution reaction: synthesis, mechanisms, and advances
Abstract
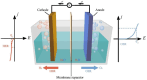
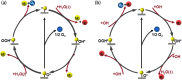
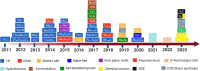
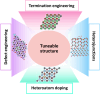
Electrochemical water splitting is a promising approach to produce H2 through renewable electricity, but its energy efficiency is severely constrained by the kinetically slow anodic oxygen evolution reaction (OER), which uses about 90% of the electricity in the water-splitting process due to its multistep proton (H+)-coupled electron (e-) transfer process, high overpotential (η), and low energy efficiency. Therefore, the quest for efficient, sustainable, and cost-effective electrocatalysts for hydrogen production through water electrolysis has intensified, highlighting the potential of two-dimensional (2D) MXenes. MXenes have emerged as a promising class of materials characterized by excellent stability, hydrophilicity, and conductivity. However, challenges such as low oxidation resistance, facile stacking, and the absence of intrinsic catalytically active sites limit their performance. This review thoroughly explores various synthesis methods for MXenes and their integration with transition metal oxides (TMOs) to tackle the challenges and enhance catalytic activity. The review also delves into advanced strategies for structural tuning of MXenes and TMOs, such as termination engineering, heteroatom doping, defect engineering, and the formation of heterojunctions. The integration of MXenes with TMOs has addressed the current limitations of MXenes and significantly boosted OER activity. By considering these structural tuning parameters and limitation factors, researchers can gain insights into the design principles and optimization strategies for MXene- and TMO-integrated electrocatalysts. The review concludes with a summary of the key findings and an outlook on future research directions, emphasizing the unexplored potential and innovative approaches that could further advance the field of electrocatalytic water splitting.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no competing financial interest.
Figures












References
-
- Bhutto Y. A. Pandey A. K. Saidur R. Laghari I. A. Buddhi D. Tyagi V. V. Investigating the optical absorption and thermal conductivity of nano-enhanced lauric acid phase change material for energy storage application. IOP Conf. Ser.: Mater. Sci. Eng. 2023;1281:012013. doi: 10.1088/1755-1315/1281/1/012013. - DOI
-
- Ali Bhutto Y. Pandey A. K. Saidur R. Laghari I. A. Khir H. Islam A. Abu Zaed M. Electrical and thermal performance assessment of photovoltaic thermal system integrated with organic phase change material. E3S Web Conf. 2024;488:01007. doi: 10.1051/e3sconf/202448801007. - DOI
-
- Ma L. Li J. Arandiyan H. Shi W. Liu C. Fu L. Influence of calcination temperature on Fe/HBEA catalyst for the selective catalytic reduction of NOx with NH3. Catal. Today. 2012;184:145–152. doi: 10.1016/j.cattod.2011.10.007. - DOI
Publication types
LinkOut - more resources
Full Text Sources

