The Revolution of Digital Therapeutics (DTx) in the Pharmaceutical Industry and Their Quality Impacts
- PMID: 39268306
- PMCID: PMC11392520
- DOI: 10.7759/cureus.66792
The Revolution of Digital Therapeutics (DTx) in the Pharmaceutical Industry and Their Quality Impacts
Abstract
An increasing number of developments and trends are driving the expansion of the digital therapeutics (DTx) market in the pharmaceutical industry. Digital therapeutics are therapies intended to treat, diagnose, and prevent diseases by using patient-directed clinically assessed software applications, which can optimize the effectiveness and delivery of healthcare. These digital innovations became important as the world changed, particularly during the coronavirus pandemic. Nowadays pharma companies are getting more comfortable with the idea of digital therapies. The majority of pharmaceutical companies are examining how to incorporate pharmaceuticals and digital therapies into their treatment regimens, leveraging digital tools to enhance patient outcomes and streamline healthcare delivery. A thorough overview of the most recent technological advancements in the creation of digital therapies shows particular technologies that are essential to the market's future growth. Moreover, the evaluation of digital therapeutics by clinical trial and real-world data is outlined. The critical quality attributes of DTx products and the challenges, including data management issues and regulatory obstacles, which make the creation, approval, and marketing of customized medicines more difficult, are covered in this review article. Overall, pharma companies are venturing into the world of digital therapeutics while acknowledging the limitations of the emerging field.
Keywords: digital health technologies (dht); digital technologies; digital therapeutics (dtx); medical device; software systems.
Copyright © 2024, Rajendran et al.
Conflict of interest statement
Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Figures
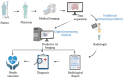
Similar articles
-
Digital Therapeutics for Improving Effectiveness of Pharmaceutical Drugs and Biological Products: Preclinical and Clinical Studies Supporting Development of Drug + Digital Combination Therapies for Chronic Diseases.J Clin Med. 2024 Jan 11;13(2):403. doi: 10.3390/jcm13020403. J Clin Med. 2024. PMID: 38256537 Free PMC article. Review.
-
AMCP Partnership Forum: Digital Therapeutics-What Are They and Where Do They Fit in Pharmacy and Medical Benefits?J Manag Care Spec Pharm. 2020 May;26(5):674-681. doi: 10.18553/jmcp.2020.19418. Epub 2020 Mar 16. J Manag Care Spec Pharm. 2020. PMID: 32175784 Free PMC article.
-
Digital therapeutics in Korea: current status, challenges, and future directions - a narrative review.J Yeungnam Med Sci. 2025;42:8. doi: 10.12701/jyms.2024.01004. Epub 2024 Nov 18. J Yeungnam Med Sci. 2025. PMID: 39551075 Free PMC article.
-
Digital Therapeutics in Hearing Healthcare: Evidence-Based Review.J Audiol Otol. 2024 Jul;28(3):159-166. doi: 10.7874/jao.2023.00780. Epub 2024 Jul 9. J Audiol Otol. 2024. PMID: 38973323 Free PMC article.
-
Co-Creation in the Development of Digital Therapeutics: A Narrative Review.Int J Environ Res Public Health. 2024 Nov 28;21(12):1589. doi: 10.3390/ijerph21121589. Int J Environ Res Public Health. 2024. PMID: 39767430 Free PMC article. Review.
Cited by
-
Digital Therapeutics for Cognitive Impairment: Exploring Innovations, Challenges, and Future Prospects.J Med Internet Res. 2025 Jun 12;27:e73689. doi: 10.2196/73689. J Med Internet Res. 2025. PMID: 40504630 Free PMC article.
References
-
- Introduction of digital therapeutics. Hong JS, Wasden C, Han DH. Comput Methods Programs Biomed. 2021;209:106319. - PubMed
-
- Digital therapeutics: an updated review. Akhtar N, Sami H, Jain A, Singhai AK. https://www.researchgate.net/publication/374558662 Inventi Rapid: NDDS. 2023;2023:1–5.
-
- Cutting-edge technologies for digital therapeutics: a review and architecture proposals for future directions. Yoo JH, Jeong H, Chung TM. Appl Sci. 2023;13:6929.
Publication types
LinkOut - more resources
Full Text Sources
