Modeling cancer-associated hypercoagulability using glioblastoma spheroids in microfluidic chips
- PMID: 39268353
- PMCID: PMC11391032
- DOI: 10.1016/j.rpth.2024.102475
Modeling cancer-associated hypercoagulability using glioblastoma spheroids in microfluidic chips
Abstract
Background: Cancer increases the risk of venous thromboembolism, and glioblastoma is one of the cancer types with the highest risk of venous thromboembolism (10%-30%). Tumor-intrinsic features are believed to affect vascular permeability and hypercoagulability, but novel models are required to study the pathophysiological dynamics underlying cancer-associated thrombosis at the molecular level.
Objectives: We have developed a novel cancer-on-a-chip model to examine the effects of glioblastoma cells on the deregulation of blood coagulation.
Methods: This was accomplished by coculturing vessel-forming human umbilical vein endothelial cells with glioblastoma spheroids overexpressing tissue factor (TF), the initiator of coagulation (U251 lentivirus, LV-TF) or an LV-control (U251 LV-Ctrl) in an OrganoPlate Graft platform.
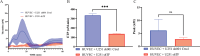
Results: Using a modified thrombin generation assay inside the cancer-on-a-chip, we found that U251 LV-Ctrl and U251 LV-TF spheroids promoted an increased procoagulant state in plasma, as was shown by a 3.1- and 7.0-fold increase in endogenous thrombin potential, respectively. Furthermore, the anticoagulant drug rivaroxaban and TF coagulation-blocking antibody 5G9 inhibited the activation of blood coagulation in U251 LV-TF spheroid-containing graft plates, as was shown by a reduced endogenous thrombin potential (4.0- and 4.4-fold, respectively).
Conclusion: With this study, we present a novel 3-dimensional cancer-on-a-chip model that has the potential to be used in the discovery of new anticoagulant drugs and identification of optimal anticoagulant strategies for glioblastoma and other cancer types.
Keywords: anticoagulants; cancer-associated thrombosis; extracellular vesicles; glioblastoma; organ-on-a-chip.
© 2024 The Author(s).
Figures















References
-
- Mulder F.I., Horváth-Puhó E., van Es N., van Laarhoven H.W.M., Pedersen L., Moik F., et al. Venous thromboembolism in cancer patients: a population-based cohort study. Blood. 2021;137:1959–1969. - PubMed
-
- Timp J.F., Braekkan S.K., Versteeg H.H., Cannegieter S.C. Epidemiology of cancer-associated venous thrombosis. Blood. 2013;122:1712–1723. - PubMed
-
- Chew H.K., Wun T., Harvey D., Zhou H., White R.H. Incidence of venous thromboembolism and its effect on survival among patients with common cancers. Arch Intern Med. 2006;166:458–464. - PubMed
-
- Khorana A.A., Francis C.W., Culakova E., Kuderer N.M., Lyman G.H. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J Thromb Haemost. 2007;5:632–634. - PubMed
-
- Rondon A.M.R., Kroone C., Kapteijn M.Y., Versteeg H.H., Buijs J.T. Role of tissue factor in tumor progression and cancer-associated thrombosis. Semin Thromb Hemost. 2019;45:396–412. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

