Conjugal plasmid transfer in the plant rhizosphere in the One Health context
- PMID: 39268528
- PMCID: PMC11390587
- DOI: 10.3389/fmicb.2024.1457854
Conjugal plasmid transfer in the plant rhizosphere in the One Health context
Abstract
Introduction: Horizontal gene transfer (HGT) of antibiotic resistance genes (ARGs) is one of the primary routes of antimicrobial resistance (AMR) dissemination. In the One Health context, tracking the spread of mobile genetic elements (MGEs) carrying ARGs in agri-food ecosystems is pivotal in understanding AMR diffusion and estimating potential risks for human health. So far, little attention has been devoted to plant niches; hence, this study aimed to evaluate the conjugal transfer of ARGs to the bacterial community associated with the plant rhizosphere, a hotspot for microbial abundance and activity in the soil. We simulated a source of AMR determinants that could enter the food chain via plants through irrigation.
Methods: Among the bacterial strains isolated from treated wastewater, the strain Klebsiella variicola EEF15 was selected as an ARG donor because of the relevance of Enterobacteriaceae in the AMR context and the One Health framework. The strain ability to recolonize lettuce, chosen as a model for vegetables that were consumed raw, was assessed by a rifampicin resistant mutant. K. variicola EEF15 was genetically manipulated to track the conjugal transfer of the broad host range plasmid pKJK5 containing a fluorescent marker gene to the natural rhizosphere microbiome obtained from lettuce plants. Transconjugants were sorted by fluorescent protein expression and identified through 16S rRNA gene amplicon sequencing.
Results and discussion: K. variicola EEF15 was able to colonize the lettuce rhizosphere and inhabit its leaf endosphere 7 days past bacterial administration. Fluorescence stereomicroscopy revealed plasmid transfer at a frequency of 10-3; cell sorting allowed the selection of the transconjugants. The conjugation rates and the strain's ability to colonize the plant rhizosphere and leaf endosphere make strain EEF15::lacIq-pLpp-mCherry-gmR with pKJK5::Plac::gfp an interesting candidate to study ARG spread in the agri-food ecosystem. Future studies taking advantage of additional environmental donor strains could provide a comprehensive snapshot of AMR spread in the One Health context.
Keywords: agri-food system; conjugation; endosphere; horizontal gene transfer; microbial community; ready-to-eat vegetables; rhizosphere.
Copyright © 2024 Riva, Dechesne, Eckert, Riva, Borin, Mapelli, Smets and Crotti.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


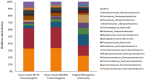
References
-
- Araújo S., Silva A. T. I., Tacão M., Patinha C., Alves A., Henriques I. (2017). Characterization of antibiotic resistant and pathogenic Escherichia coli in irrigation water and vegetables in household farms. Int. J. Food Microbiol. 257, 192–200. doi: 10.1016/j.ijfoodmicro.2017.06.020, PMID: - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

