RTP801 interacts with the tRNA ligase complex and dysregulates its RNA ligase activity in Alzheimer's disease
- PMID: 39268577
- PMCID: PMC11472047
- DOI: 10.1093/nar/gkae776
RTP801 interacts with the tRNA ligase complex and dysregulates its RNA ligase activity in Alzheimer's disease
Abstract
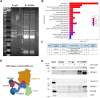
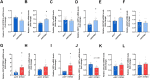
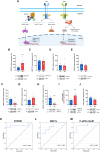
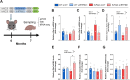
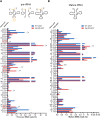
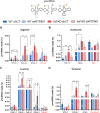
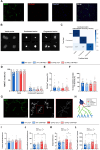
RTP801/REDD1 is a stress-responsive protein overexpressed in neurodegenerative diseases such as Alzheimer's disease (AD) that contributes to cognitive deficits and neuroinflammation. Here, we found that RTP801 interacts with HSPC117, DDX1 and CGI-99, three members of the tRNA ligase complex (tRNA-LC), which ligates the excised exons of intron-containing tRNAs and the mRNA exons of the transcription factor XBP1 during the unfolded protein response (UPR). We also found that RTP801 modulates the mRNA ligase activity of the complex in vitro since RTP801 knockdown promoted XBP1 splicing and the expression of its transcriptional target, SEC24D. Conversely, RTP801 overexpression inhibited the splicing of XBP1. Similarly, in human AD postmortem hippocampal samples, where RTP801 is upregulated, we found that XBP1 splicing was dramatically decreased. In the 5xFAD mouse model of AD, silencing RTP801 expression in hippocampal neurons promoted Xbp1 splicing and prevented the accumulation of intron-containing pre-tRNAs. Finally, the tRNA-enriched fraction obtained from 5xFAD mice promoted abnormal dendritic arborization in cultured hippocampal neurons, and RTP801 silencing in the source neurons prevented this phenotype. Altogether, these results show that elevated RTP801 impairs RNA processing in vitro and in vivo in the context of AD and suggest that RTP801 inhibition could be a promising therapeutic approach.
© The Author(s) 2024. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures


References
-
- Ellisen L.W., Ramsayer K.D., Johannessen C.M., Yang A., Beppu H., Minda K., Oliner J.D., McKeon F., Haber D.A.. REDD1, a developmentally regulated transcriptional target of p63 and p53, links p63 to regulation of reactive oxygen species. Mol. Cell. 2002; 10:995–1005. - PubMed
-
- Kim J.R., Lee S.R., Chung H.J., Kim S., Baek S.H., Kim J.H., Kim Y.S.. Identification of amyloid β-peptide responsive genes by cDNA microarray technology: involvement of RTP801 in amyloid β-peptide toxicity. Exp. Mol. Med. 2003; 35:403–411. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

