Entrapment of antimicrobial compounds in a metal matrix for crop protection
- PMID: 39268832
- PMCID: PMC11393771
- DOI: 10.1111/1751-7915.70005
Entrapment of antimicrobial compounds in a metal matrix for crop protection
Abstract
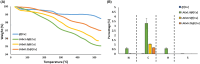
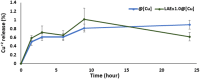
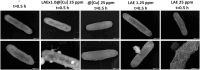
Agricultural yields are often limited by damage caused by pathogenic microorganisms, including plant-pathogenic bacteria. The chemical control options to cope with bacterial diseases in agriculture are limited, predominantly relying on copper-based products. These compounds, however, possess limited efficacy. Therefore, there is an urgent need to develop novel technologies to manage bacterial plant diseases and reduce food loss. In this study, a new antimicrobial agent was developed using a doping method that entraps small bioactive organic molecules inside copper as the metal matrix. The food preservative agent lauroyl arginate ethyl ester (ethyl lauroyl arginate; LAE) was chosen as the doped organic compound. The new composites were termed LAE@[Cu]. Bactericidal assays against Acidovorax citrulli, a severe plant pathogen, revealed that LAE and copper in the composites possess a synergistic interaction as compared with each component individually. LAE@[Cu] composites were further characterised in terms of chemical properties and in planta assays demonstrated their potential for further development as crop protection agents.
© 2024 The Author(s). Microbial Biotechnology published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interests.
Figures



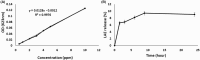

References
-
- Antonoglou, O. , Giannousi, K. , Arvanitidis, J. , Mourdikoudis, S. , Pantazaki, A. & Dendrinou‐Samara, C. (2017) Elucidation of one step synthesis of PEGylated CuFe bimetallic nanoparticles. Antimicrobial activity of CuFe@PEG vs Cu@PEG. Journal of Inorganic Biochemistry, 177, 159–170. - PubMed
-
- Asker, D. , Weiss, J. & McClements, D.J. (2009) Analysis of the interactions of a cationic surfactant (Lauric Arginate) with an anionic biopolymer (pectin): isothermal titration calorimetry, light scattering, and microelectrophoresis. Langmuir, 25, 116–122. - PubMed
-
- Avnir, D. (2014) Molecularly doped metals. Accounts of Chemical Research, 47(2), 579–592. - PubMed
-
- Bahar, O. & Burdman, S. (2010) Bacterial fruit blotch: a threat to the cucurbit industry. Israel Journal of Plant Sciences, 58, 19–31.
-
- Bahar, O. , Kritzman, G. & Burdman, S. (2009) Bacterial fruit blotch of melon: screens for disease tolerance and role of seed transmission in pathogenicity. European Journal of Plant Pathology, 123, 71–83.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

