CaMKK2: bridging the gap between Ca2+ signaling and energy-sensing
- PMID: 39268917
- PMCID: PMC11576191
- DOI: 10.1042/EBC20240011
CaMKK2: bridging the gap between Ca2+ signaling and energy-sensing
Abstract
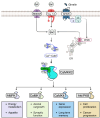
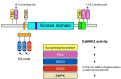
Calcium (Ca2+) ions are ubiquitous and indispensable signaling messengers that regulate virtually every cell function. The unique ability of Ca2+ to regulate so many different processes yet cause stimulus specific changes in cell function requires sensing and decoding of Ca2+ signals. Ca2+-sensing proteins, such as calmodulin, decode Ca2+ signals by binding and modifying the function of a diverse range of effector proteins. These effectors include the Ca2+-calmodulin dependent protein kinase kinase-2 (CaMKK2) enzyme, which is the core component of a signaling cascade that plays a key role in important physiological and pathophysiological processes, including brain function and cancer. In addition to its role as a Ca2+ signal decoder, CaMKK2 also serves as an important junction point that connects Ca2+ signaling with energy metabolism. By activating the metabolic regulator AMP-activated protein kinase (AMPK), CaMKK2 integrates Ca2+ signals with cellular energy status, enabling the synchronization of cellular activities regulated by Ca2+ with energy availability. Here, we review the structure, regulation, and function of CaMKK2 and discuss its potential as a treatment target for neurological disorders, metabolic disease, and cancer.
Keywords: AMPK; CaMKK2; calcium signaling; calmodulin; energy-sensing.
© 2024 The Author(s).
Conflict of interest statement
M.A.F. is a founder and shareholder of Celesta Therapeutics. The authors declare that this review was prepared in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

