Hypophosphatemia Correction Reduces ICANS Incidence and Duration in CAR T-cell Therapy: A Pooled Clinical Trial Analysis
- PMID: 39269033
- PMCID: PMC11448391
- DOI: 10.1158/2767-9764.CRC-24-0250
Hypophosphatemia Correction Reduces ICANS Incidence and Duration in CAR T-cell Therapy: A Pooled Clinical Trial Analysis
Abstract
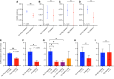
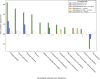
A common complication of chimeric antigen receptor (CAR) T-cell therapy is immune effector cell-associated neurotoxicity syndrome (ICANS), which presents with encephalopathy, aphasia, inattention, somnolence, seizures, weakness, or cerebral edema. Despite its significant morbidity, there are currently no effective targeted treatments. Given the clinical similarities between ICANS and the neurological manifestations of acute hypophosphatemia, we retrospectively reviewed 499 patients treated with CD19-targeted CAR T-cell therapy across multiple clinical trials between 2015 and 2020. In addition to clinical toxicities experienced by the patients, we also interrogated the impact of serum electrolyte data and repletion of corresponding electrolyte deficiencies with ICANS incidence, severity, and duration. Hypophosphatemia was a common occurrence in CAR T-cell recipients and the only electrolyte derangement associated with a significantly higher cumulative incidence of ICANS. Moreover, phosphorus repletion in patients with hypophosphatemia was associated with significantly decreased ICANS incidence and duration. Hypophosphatemia was uniquely associated with encephalopathy neurological adverse events, which also showed the strongest positive correlation with both ICANS and cytokine release syndrome severity. These findings suggest that serum phosphorus could be a reliable biomarker for ICANS, and expeditious, goal-directed phosphorus repletion in response to serum hypophosphatemia could be a safe, inexpensive, and widely available intervention for such patients.
Significance: Herein we show that phosphorus repletion in patients with hypophosphatemia receiving anti-CD19 chimeric antigen receptor T-cell therapeutics was associated with significantly decreased immune effector cell-associated neurotoxicity syndrome (ICANS) incidence and symptom duration. Given the significant morbidity associated with ICANS and lack of targeted interventions, hypophosphatemia may serve as both a useful biomarker and an inexpensive intervention for ICANS.
©2024 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
P. Lafeuille reports other support from Medidata AI, a Dassault Systemes company, during the conduct of the study. S.S. Diamond reports other support from Dassault Systemes during the conduct of the study, as well as other support from Brigham and Women’s Hospital outside the submitted work. M.R. Hanudel reports personal fees from GlaxoSmithKline and Mead Johnson Nutrition outside the submitted work. T.S. Nowicki reports personal fees from Medidata during the conduct of the study, as well as personal fees from Allogene Therapeutics, PACT Pharma, and Adaptive Biotechnologies outside the submitted work. No disclosures were reported by the other authors.
Figures