Lymphoid origin of intrinsically activated plasmacytoid dendritic cells in mice
- PMID: 39269281
- PMCID: PMC11398865
- DOI: 10.7554/eLife.96394
Lymphoid origin of intrinsically activated plasmacytoid dendritic cells in mice
Abstract
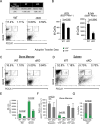
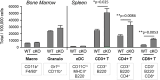
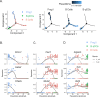
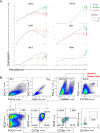
We identified a novel mouse plasmacytoid dendritic cell (pDC) lineage derived from the common lymphoid progenitors (CLPs) that is dependent on expression of Bcl11a. These CLP-derived pDCs, which we refer to as 'B-pDCs', have a unique gene expression profile that includes hallmark B cell genes, normally not expressed in conventional pDCs. Despite expressing most classical pDC markers such as SIGLEC-H and PDCA1, B-pDCs lack IFN-α secretion, exhibiting a distinct inflammatory profile. Functionally, B-pDCs induce T cell proliferation more robustly than canonical pDCs following Toll-like receptor 9 (TLR9) engagement. B-pDCs, along with another homogeneous subpopulation of myeloid-derived pDCs, display elevated levels of the cell surface receptor tyrosine kinase AXL, mirroring human AXL+ transitional DCs in function and transcriptional profile. Murine B-pDCs therefore represent a phenotypically and functionally distinct CLP-derived DC lineage specialized in T cell activation and previously not described in mice.
Keywords: B cells; CLPs; immunology; inflammation; mouse; plasmacytoid DCs.
© 2024, Araujo, Dekker et al.
Conflict of interest statement
AA, JD, KG, ZS, CR, ZH, BL, DO, JL, VI, LE, GG, GI, SY, HT No competing interests declared
Figures







Update of
- doi: 10.1101/310680
- doi: 10.7554/eLife.96394.1
- doi: 10.7554/eLife.96394.2
References
-
- Alcántara-Hernández M, Leylek R, Wagar LE, Engleman EG, Keler T, Marinkovich MP, Davis MM, Nolan GP, Idoyaga J. High-dimensional phenotypic mapping of human dendritic cells reveals interindividual variation and tissue specialization. Immunity. 2017;47:1037–1050. doi: 10.1016/j.immuni.2017.11.001. - DOI - PMC - PubMed
-
- Björck P, Kincade PW. CD19+ pro-B cells can give rise to dendritic cells in vitro. Journal of Immunology. 1998;161:5795–5799. - PubMed
-
- Cisse B, Caton ML, Lehner M, Maeda T, Scheu S, Locksley R, Holmberg D, Zweier C, den Hollander NS, Kant SG, Holter W, Rauch A, Zhuang Y, Reizis B. Transcription factor E2-2 is an essential and specific regulator of plasmacytoid dendritic cell development. Cell. 2008;135:37–48. doi: 10.1016/j.cell.2008.09.016. - DOI - PMC - PubMed
MeSH terms
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
- The Lymphoma Research Foundation Fellowship 300463/LRF/Lymphoma Research Foundation/United States
- Maximizing Investigators' Research Award (MIRA) (R35) R35GM133658/NIH Office of the Director
- R01 CA130075/CA/NCI NIH HHS/United States
- F32 CA110624/CA/NCI NIH HHS/United States
- NIH Research Project Grant Program (R01) R01AI104870/NIH Office of the Director
- Endowment/Marie Betzner Morrow Centennial Endowment
- RP120459/CPRIT
- R01 CA031534/CA/NCI NIH HHS/United States
- R35 GM133658/GM/NIGMS NIH HHS/United States
- CPRIT RP120459/Cancer Prevention and Research Institute of Texas
- Ruth L. Kirschstein Postdoctoral Individual National Research Service Award F32CA110624/NIH Office of the Director
- NIH Research Project Grant Program (R01) R01CA130075/NIH Office of the Director
- R01 AI104870/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

